线粒体靶向铱(III)-PF-06840003偶联物:凋亡诱导、IDO抑制和ICD反应。
IF 3.2
2区 化学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
吲哚胺2,3-双加氧酶(IDO)是肿瘤免疫治疗的潜在靶点。越来越多的证据表明IDO抑制剂可与化疗药物发挥协同作用。本研究通过IDO抑制剂PF-06840003 (PF)与Ir(III)和Ru(II)配合物偶联,合成了六个配合物,包括三个铱(III)配合物Ir-PF-1-3和三个钌(II)配合物Ru-PF-1-3。其中,铱(III)配合物Ir-PF-1-3对多种癌细胞,尤其是HeLa人宫颈癌细胞表现出优异的细胞毒性。Ir-PF-1-3具有抑制HeLa细胞迁移和集落形成的能力,显示出强大的抗转移特性。此外,Ir-PF-1-3可在细胞环境中水解并释放IDO抑制活性成分PF-06840003,发挥IDO抑制作用。同时,Ir-PF-1-3主要位于线粒体,破坏线粒体的结构和功能。这表现为线粒体膜电位(MMP)的降低和活性氧(ROS)水平的增加。在细胞凋亡和免疫原性细胞死亡(ICD)诱导过程中,也存在G2/M期周期阻滞。本文章由计算机程序翻译,如有差异,请以英文原文为准。
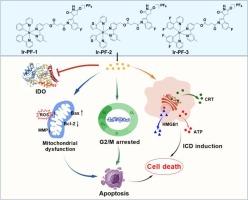
Mitochondria-targeted iridium(III)-PF-06840003 conjugates: Apoptosis induction, IDO inhibition and ICD response
Indoleamine 2,3-dioxygenase (IDO) is a potential target for tumor immunotherapy. The growing evidence suggests that IDO inhibitors can exert synergistic effects with chemotherapy drugs. In this study, six complexes, comprising three iridium(III) complexes Ir-PF-1–3 and three ruthenium(II) complexes Ru-PF-1–3, were synthesized through the coupling of an IDO inhibitor PF-06840003 (PF) with Ir(III) and Ru(II) complexes. Among them, iridium(III) complexes Ir-PF-1–3 exhibited excellent cytotoxicity against various cancer cells, especially HeLa human cervical cancer cells. Ir-PF-1–3 exhibited potent anti-metastatic properties, as evidenced by their ability to inhibit the migration and colony formation of HeLa cells. Furthermore, Ir-PF-1–3 could be hydrolyzed in the cellular environment and released the IDO inhibitory active component PF-06840003, exerting an IDO inhibitory effect. Meanwhile, Ir-PF-1–3 mainly located in mitochondria, where they disrupt mitochondrial structure and function. This is manifested by a decrease in mitochondrial membrane potential (MMP) and an increase in reactive oxygen species (ROS) levels. During apoptosis and immunogenic cell death (ICD) induction, there is also G2/M phase cycle arrest.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Inorganic Biochemistry
生物-生化与分子生物学
CiteScore
7.00
自引率
10.30%
发文量
336
审稿时长
41 days
期刊介绍:
The Journal of Inorganic Biochemistry is an established international forum for research in all aspects of Biological Inorganic Chemistry. Original papers of a high scientific level are published in the form of Articles (full length papers), Short Communications, Focused Reviews and Bioinorganic Methods. Topics include: the chemistry, structure and function of metalloenzymes; the interaction of inorganic ions and molecules with proteins and nucleic acids; the synthesis and properties of coordination complexes of biological interest including both structural and functional model systems; the function of metal- containing systems in the regulation of gene expression; the role of metals in medicine; the application of spectroscopic methods to determine the structure of metallobiomolecules; the preparation and characterization of metal-based biomaterials; and related systems. The emphasis of the Journal is on the structure and mechanism of action of metallobiomolecules.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


