人β2肾上腺素能受体失活的恒ph模拟
IF 5.3
2区 化学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
了解ph依赖性G蛋白偶联受体(GPCR)信号传导的分子基础对于理解生理调控和药物设计至关重要。在这里,我们研究了人β2肾上腺素能受体(β2AR),这是一种典型的GPCR,其功能对pH条件敏感。通过广泛的恒定pH分子动力学模拟,我们提供了β2AR在生理相关pH值下失活的详细原子表征(4-9)。我们的模拟表明,β2AR失活与关键残基的质子化事件密切相关,特别是E2686×30参与了离子锁的形成。此外,我们发现在没有钠直接结合到残基周围的离子结合袋D792×50的情况下发生失活。相反,钠离子主要与D1133×32相互作用,有效地阻止更深的进入传统的结合位点。这些结果挑战了现有的机制模型,并强调了在GPCR模拟中准确建模静电的必要性。我们的研究结果强调了恒定ph方法的潜力,以促进对GPCR动力学的理解,影响基础生物学和治疗策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
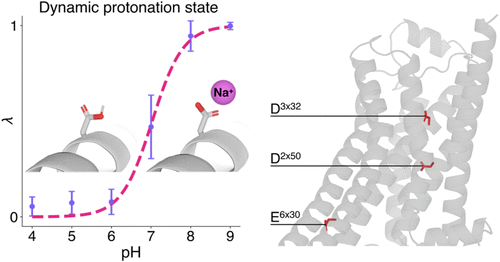
Constant-pH Simulation of the Human β2 Adrenergic Receptor Inactivation
Understanding the molecular basis of pH-dependent G protein-coupled receptor (GPCR) signaling is crucial for comprehending physiological regulation and drug design. Here, we investigate the human β2 adrenergic receptor (β2AR), a prototypical GPCR whose function is sensitive to pH conditions. Employing extensive constant-pH molecular dynamics simulations, we provide a detailed atomistic characterization of β2AR inactivation across physiologically relevant pH values (4–9). Our simulations reveal that β2AR inactivation is closely linked to protonation events at critical residues, notably E2686×30 involved in the ionic lock formation. Furthermore, we find that inactivation occurs without direct sodium binding to the ion-binding pocket around residue D792×50. Instead, sodium ions predominantly interact with D1133×32, effectively blocking deeper entry toward the traditional binding site. These results challenge existing mechanistic models and highlight the necessity of accurately modeling electrostatics in GPCR simulations. Our findings underscore the potential of constant-pH methodologies to advance the understanding of GPCR dynamics, influencing both fundamental biology and therapeutic strategies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.80
自引率
10.70%
发文量
529
审稿时长
1.4 months
期刊介绍:
The Journal of Chemical Information and Modeling publishes papers reporting new methodology and/or important applications in the fields of chemical informatics and molecular modeling. Specific topics include the representation and computer-based searching of chemical databases, molecular modeling, computer-aided molecular design of new materials, catalysts, or ligands, development of new computational methods or efficient algorithms for chemical software, and biopharmaceutical chemistry including analyses of biological activity and other issues related to drug discovery.
Astute chemists, computer scientists, and information specialists look to this monthly’s insightful research studies, programming innovations, and software reviews to keep current with advances in this integral, multidisciplinary field.
As a subscriber you’ll stay abreast of database search systems, use of graph theory in chemical problems, substructure search systems, pattern recognition and clustering, analysis of chemical and physical data, molecular modeling, graphics and natural language interfaces, bibliometric and citation analysis, and synthesis design and reactions databases.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


