含4,4′-联吡啶的一维有机金属镧系化合物的磁滞现象
IF 7.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
装配带有高度各向异性构件的多核配合物仍然是开发先进功能材料的一种有吸引力的方法。然而,将基于镧系金属的新铈组分[CpR2Ln]+结合到高阶体系中仍然是一个重大的合成挑战,而且它们的靶向分离非常罕见。本文介绍的有机金属镧系化合物链承载桥接4,4 ' -联吡啶配体,(其中Ln = Gd (1), Tb (2), Dy (3);五甲基环戊二烯;Bpy = 4,4 ' -联吡啶)。这构成了通过有机桥相互连接的镧系金属单元的晶体学表征的一维有机金属网络的第一份报告。每个茂金属基团由两个联吡啶配体连接,形成之字形链,其中四苯基硼酸盐阴离子位于氮配体之间。和4,4′-联吡啶的化合物形成非常快,导致在极性溶剂THF中立即沉淀。因此,开发了一种明智的合成路线,以确保结晶和纯分离,其中涉及使用h管。1 - 3的直流磁化率测量表明存在未偶联的镧系离子,这与实验cw-EPR谱以及通过破对称DFT计算得到的钆同系物1的磁交换耦合常数J一致。镝类似物3是一种单分子磁体(SMM),通过在零直流场作用下的失相交流磁化率信号(表明磁弛豫缓慢)和等温变场直流测量(显示高达8 K的开放磁滞回线)证实了这一点。缺乏链内和链间的磁性交换表明,3中单分子磁性的来源是单离子各向异性和晶体场,这一点通过从头算进一步得到了支持。本文章由计算机程序翻译,如有差异,请以英文原文为准。
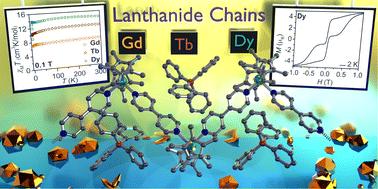
Magnetic hysteresis in 1D organometallic lanthanide chain compounds containing 4,4′-bipyridine
The assembly of multinuclear complexes bearing highly anisotropic building blocks remains an attractive approach to developing advanced functional materials. However, incorporating lanthanide-based metallocenium moieties, [CpR2Ln]+, into higher-order systems remains a significant synthetic challenge and their targeted isolation is exceedingly rare. Presented herein are organometallic lanthanide chain compounds bearing bridging 4,4′-bipyridine ligands, 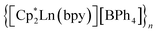 (where Ln = Gd (1), Tb (2), Dy (3); Cp* = pentamethylcyclopentadienyl; bpy = 4,4′-bipyridine). This constitutes the first report of a crystallographically characterised 1D organometallic network of lanthanide metallocenium units connected to one another through organic bridges. Each metallocenium moiety is ligated by two bipyridyl ligands, giving rise to zigzag-shaped chains, where tetraphenylborate anions reside in between the nitrogen ligands. The formation of the compounds from
(where Ln = Gd (1), Tb (2), Dy (3); Cp* = pentamethylcyclopentadienyl; bpy = 4,4′-bipyridine). This constitutes the first report of a crystallographically characterised 1D organometallic network of lanthanide metallocenium units connected to one another through organic bridges. Each metallocenium moiety is ligated by two bipyridyl ligands, giving rise to zigzag-shaped chains, where tetraphenylborate anions reside in between the nitrogen ligands. The formation of the compounds from 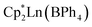 and 4,4′-bipyridine is very fast, leading to an immediate precipitation in the polar solvent THF. Thus, a judicious synthetic route was developed to ensure crystallisation and pure isolation which involved the use of an H-tube. Dc magnetic susceptibility measurements for 1–3 allude to the presence of uncoupled lanthanide ions, which is consistent with the experimental cw-EPR spectrum as well as the calculated magnetic exchange coupling constant, J, for the gadolinium congener, 1, obtained through broken-symmetry DFT. The dysprosium analogue, 3, is a single-molecule magnet (SMM) which was confirmed through both out-of-phase ac magnetic susceptibility signals under a zero applied dc field, indicative of slow magnetic relaxation, and isothermal, variable-field dc measurements, revealing open magnetic hysteresis loops up to 8 K. The lack of intra- and interchain magnetic exchange suggests that the origin of single-molecule magnetism in 3 arises from single-ion anisotropy and crystal field, which is further supported via ab initio calculations.
and 4,4′-bipyridine is very fast, leading to an immediate precipitation in the polar solvent THF. Thus, a judicious synthetic route was developed to ensure crystallisation and pure isolation which involved the use of an H-tube. Dc magnetic susceptibility measurements for 1–3 allude to the presence of uncoupled lanthanide ions, which is consistent with the experimental cw-EPR spectrum as well as the calculated magnetic exchange coupling constant, J, for the gadolinium congener, 1, obtained through broken-symmetry DFT. The dysprosium analogue, 3, is a single-molecule magnet (SMM) which was confirmed through both out-of-phase ac magnetic susceptibility signals under a zero applied dc field, indicative of slow magnetic relaxation, and isothermal, variable-field dc measurements, revealing open magnetic hysteresis loops up to 8 K. The lack of intra- and interchain magnetic exchange suggests that the origin of single-molecule magnetism in 3 arises from single-ion anisotropy and crystal field, which is further supported via ab initio calculations.
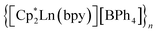 (where Ln = Gd (1), Tb (2), Dy (3); Cp* = pentamethylcyclopentadienyl; bpy = 4,4′-bipyridine). This constitutes the first report of a crystallographically characterised 1D organometallic network of lanthanide metallocenium units connected to one another through organic bridges. Each metallocenium moiety is ligated by two bipyridyl ligands, giving rise to zigzag-shaped chains, where tetraphenylborate anions reside in between the nitrogen ligands. The formation of the compounds from
(where Ln = Gd (1), Tb (2), Dy (3); Cp* = pentamethylcyclopentadienyl; bpy = 4,4′-bipyridine). This constitutes the first report of a crystallographically characterised 1D organometallic network of lanthanide metallocenium units connected to one another through organic bridges. Each metallocenium moiety is ligated by two bipyridyl ligands, giving rise to zigzag-shaped chains, where tetraphenylborate anions reside in between the nitrogen ligands. The formation of the compounds from 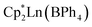 and 4,4′-bipyridine is very fast, leading to an immediate precipitation in the polar solvent THF. Thus, a judicious synthetic route was developed to ensure crystallisation and pure isolation which involved the use of an H-tube. Dc magnetic susceptibility measurements for 1–3 allude to the presence of uncoupled lanthanide ions, which is consistent with the experimental cw-EPR spectrum as well as the calculated magnetic exchange coupling constant, J, for the gadolinium congener, 1, obtained through broken-symmetry DFT. The dysprosium analogue, 3, is a single-molecule magnet (SMM) which was confirmed through both out-of-phase ac magnetic susceptibility signals under a zero applied dc field, indicative of slow magnetic relaxation, and isothermal, variable-field dc measurements, revealing open magnetic hysteresis loops up to 8 K. The lack of intra- and interchain magnetic exchange suggests that the origin of single-molecule magnetism in 3 arises from single-ion anisotropy and crystal field, which is further supported via ab initio calculations.
and 4,4′-bipyridine is very fast, leading to an immediate precipitation in the polar solvent THF. Thus, a judicious synthetic route was developed to ensure crystallisation and pure isolation which involved the use of an H-tube. Dc magnetic susceptibility measurements for 1–3 allude to the presence of uncoupled lanthanide ions, which is consistent with the experimental cw-EPR spectrum as well as the calculated magnetic exchange coupling constant, J, for the gadolinium congener, 1, obtained through broken-symmetry DFT. The dysprosium analogue, 3, is a single-molecule magnet (SMM) which was confirmed through both out-of-phase ac magnetic susceptibility signals under a zero applied dc field, indicative of slow magnetic relaxation, and isothermal, variable-field dc measurements, revealing open magnetic hysteresis loops up to 8 K. The lack of intra- and interchain magnetic exchange suggests that the origin of single-molecule magnetism in 3 arises from single-ion anisotropy and crystal field, which is further supported via ab initio calculations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Science
CHEMISTRY, MULTIDISCIPLINARY-
CiteScore
14.40
自引率
4.80%
发文量
1352
审稿时长
2.1 months
期刊介绍:
Chemical Science is a journal that encompasses various disciplines within the chemical sciences. Its scope includes publishing ground-breaking research with significant implications for its respective field, as well as appealing to a wider audience in related areas. To be considered for publication, articles must showcase innovative and original advances in their field of study and be presented in a manner that is understandable to scientists from diverse backgrounds. However, the journal generally does not publish highly specialized research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


