再论LixCoO2和NaxCoO2的离子动力学
IF 7.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
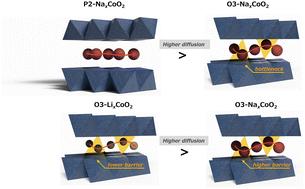
层状氧化物(AMO2,其中A = Li或Na, M =过渡金属)是锂离子和钠离子电池必不可少的正极材料。离子输运的一个基本问题是Li+和Na+在这些材料中扩散得更快;然而,从颗粒大小和电极组成的影响中区分本然扩散特性是具有挑战性的。利用操作子自旋光谱和分子动力学模拟,测定了O3-LixCoO2、O3-NaxCoO2和P2-NaxCoO2中Li+和Na+的自扩散系数。我们的研究结果表明,在p2型结构中Na+的扩散比在o3型结构中更高,这主要是由于较弱的静电相互作用。在o3型结构中,Li+的扩散速度比Na+快,而Na+的离子尺寸较大阻碍了迁移率。这些见解阐明了离子传输机制,并推动了下一代电池材料的设计。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Revisiting the ion dynamics in LixCoO2 and NaxCoO2
Layered oxides (AMO2, where A = Li or Na and M = transition metal) are essential positive electrode materials for lithium- and sodium-ion batteries. A fundamental question in ion transport is whether Li+ or Na+ diffuses faster in these materials; however, distinguishing intrinsic diffusion properties from the effects of particle size and electrode composition is challenging. Using operando muon spin spectroscopy and molecular dynamics simulations, we determined the Li+ and Na+ self-diffusion coefficients in O3-LixCoO2, O3-NaxCoO2, and P2-NaxCoO2. Our findings revealed that Na+ diffusion is higher in the P2-type structure than in the O3-type structure primarily due to weaker electrostatic interactions. In the O3-type structure, Li+ diffuses faster than Na+, whose larger ionic size hinders mobility. These insights clarify the ion transport mechanisms and advance the design of next-generation battery materials.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Science
CHEMISTRY, MULTIDISCIPLINARY-
CiteScore
14.40
自引率
4.80%
发文量
1352
审稿时长
2.1 months
期刊介绍:
Chemical Science is a journal that encompasses various disciplines within the chemical sciences. Its scope includes publishing ground-breaking research with significant implications for its respective field, as well as appealing to a wider audience in related areas. To be considered for publication, articles must showcase innovative and original advances in their field of study and be presented in a manner that is understandable to scientists from diverse backgrounds. However, the journal generally does not publish highly specialized research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


