Sirtuin 6驱动的ASC去乙酰化具有肝保护作用:一种有前途的实验性NASH炎症小体抑制方法。
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
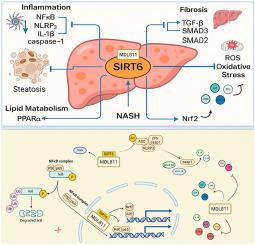
非酒精性脂肪性肝炎(NASH)是代谢性脂肪变性肝病(MASLD)的一种进行性亚型,以肝细胞损伤、炎症、氧化应激和纤维化为特征。SIRT6是一种去乙酰化酶,在调节肝脏代谢和炎症途径中起着至关重要的作用。本研究评估了MDL811(一种选择性SIRT6激活剂)在二乙基亚硝胺、高脂肪饮食和硫乙酰胺诱导的NASH大鼠模型中的治疗潜力和潜在机制。大鼠单独使用MDL811或与SIRT6抑制剂SIRT6- in -1联合治疗。MDL811显著改善肝功能指标、脂质谱和抗氧化酶活性,同时减少肝脂肪变性、炎症和纤维化。机制上,MDL811通过降低炎性小体接头ASC的乙酰化,抑制NLRP3炎性小体的激活,降低核NFκB p65活性和下游细胞因子表达,增强肝脏SIRT6活性并发挥抗炎作用。同时,MDL811通过抑制SMAD2/3磷酸化和下调α-SMA、TGF-β1、TIMP-1等纤维化标志物来减轻纤维化,同时恢复PPARα的表达。与SIRT6- in -1联合治疗消除了MDL811对炎症、纤维化、氧化应激和脂质代谢的有益作用,证实了SIRT6激活的核心作用。值得注意的是,SIRT6- in -1在对照动物中单独降低了肝脏SIRT6活性,而没有引起显著的病理,但在NASH背景下选择性地恶化了炎症和纤维化途径。这些发现表明MDL811通过sirt6依赖性抑制炎性小体信号传导来预防NASH, ASC去乙酰化被认为是一个关键机制。因此,调节SIRT6-ASC轴可能是治疗炎症驱动疾病(如NASH)的一种有希望的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Sirtuin 6-driven ASC deacetylation confers hepatoprotection: A promising approach to inflammasome inhibition in experimental NASH
Nonalcoholic steatohepatitis (NASH), a progressive subtype of metabolic-associated steatotic liver disease (MASLD), is characterized by hepatocellular injury, inflammation, oxidative stress, and fibrosis. Sirtuin 6 (SIRT6), a deacetylase, plays a crucial role in regulating hepatic metabolic and inflammatory pathways. This work assessed the therapeutic potential and underlying mechanism of MDL811, a selective SIRT6 activator, in a rat model of NASH induced by diethylnitrosamine, high-fat diet, and thioacetamide. Rats were treated with MDL811 alone or in combination with the SIRT6 inhibitor SIRT6-IN-1. MDL811 significantly improved liver function markers, lipid profile, and antioxidant enzyme activity, while reducing hepatic steatosis, inflammation, and fibrosis. Mechanistically, MDL811 enhanced hepatic SIRT6 activity and exerted anti-inflammatory effects by reducing acetylation of the inflammasome adaptor ASC, suppressing NLRP3 inflammasome activation, and decreasing nuclear NFκB p65 activity and downstream cytokine expression. In parallel, MDL811 attenuated fibrosis by inhibiting SMAD2/3 phosphorylation and downregulating fibrotic markers such as α-SMA, TGF-β1, and TIMP-1, while restoring PPARα expression. Co-treatment with SIRT6-IN-1 abolished the beneficial effects of MDL811 on inflammation, fibrosis, oxidative stress, and lipid metabolism, confirming the central role of SIRT6 activation. Notably, SIRT6-IN-1 alone reduced hepatic SIRT6 activity in control animals without inducing significant pathology, but selectively worsened inflammatory and fibrogenic pathways in the NASH context. These findings show that MDL811 protects against NASH through SIRT6-dependent inhibition of inflammasome signaling, with ASC deacetylation identified as a key mechanism. Modulating the SIRT6–ASC axis may therefore represent a promising strategy for managing inflammation-driven diseases such as NASH.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.00
自引率
0.00%
发文量
572
审稿时长
34 days
期刊介绍:
The European Journal of Pharmacology publishes research papers covering all aspects of experimental pharmacology with focus on the mechanism of action of structurally identified compounds affecting biological systems.
The scope includes:
Behavioural pharmacology
Neuropharmacology and analgesia
Cardiovascular pharmacology
Pulmonary, gastrointestinal and urogenital pharmacology
Endocrine pharmacology
Immunopharmacology and inflammation
Molecular and cellular pharmacology
Regenerative pharmacology
Biologicals and biotherapeutics
Translational pharmacology
Nutriceutical pharmacology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


