白细胞介素13通过促进周细胞自噬减轻外伤性脑损伤。
IF 8.2
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
白细胞介素13 (Interleukin 13, IL13)是趋化因子家族中的一种细胞因子,具有多种复杂的生理作用。以往的研究一致发现,它通过免疫调节和影响神经炎症,在包括创伤性脑损伤(TBI)在内的各种类型的神经损伤和神经退行性疾病中发挥重要作用。然而,对创伤性脑损伤后的其他生理影响知之甚少。在这项研究中,我们发现了IL13之前未被报道的重要作用。在小鼠TBI模型中,我们发现IL13可以以剂量依赖的方式保护周细胞的损失,并且这种作用在两个与TBI相关的涉及周细胞的体外损伤模型中得到了验证。中和IL13水平显著逆转了这种保护作用,突出了IL13在TBI后保护周细胞中的重要性。此外,通过转录组分析结合药理学方法,体内和体外实验均表明,IL13通过促进周细胞自噬发挥这种保护作用,这对于IL13诱导的TBI后血管生成和神经保护至关重要。此外,我们的机制研究表明,IL13通过激活SIRT1 /叉头盒O3自噬途径发挥这种保护作用。这一发现为研究IL13在神经损伤中的新调控机制提供了新的见解,并强调了周细胞在TBI中的关键作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
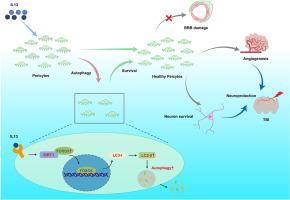
Interleukin 13 alleviates traumatic brain injury by promoting pericyte autophagy
Interleukin 13 (IL13), as a cytokine belonging to the chemokine family, exhibits multiple complex physiological effects. Previous studies have consistently observed its significant role in various types of neural injuries and neurodegenerative diseases, including traumatic brain injury (TBI), through immune modulation and influencing neuroinflammation. However, little is known about its other physiological effects following TBI. In this study, we discovered a previously unreported important effect of IL13. In mouse models of TBI, we found that IL13 can protect against the loss of pericytes in a dose-dependent manner, and this effect was validated in two in vitro injury models relevant to TBI involving pericytes. Neutralizing IL13 levels significantly reversed this protective effect, highlighting the importance of IL13 in protecting pericytes after TBI. Further, through transcriptome analysis combined with pharmacological methods, both in vivo and in vitro experiments revealed that IL13 exerts this protective effect by promoting pericytes autophagy, which is crucial for IL13-induced angiogenesis and neuroprotection after TBI. Additionally, our mechanistic studies revealed that IL13 exerts this protective effect by activating the sirtuin 1 (SIRT1)/the forkhead box O3 (FOXO3) autophagy pathway. This finding provides new insights into the investigation of novel regulatory mechanisms of IL13 in neural injuries and highlights the crucial role of pericytes in TBI.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


