设计具有超强偶极矩的小有机化合物
IF 3.1
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
在反应性的引导下,通过给电子基团(- nh2)和吸电子基团(-CN)取代,Azulene的偶极矩得到增强。衍生品被分为四类。DFT计算显示,环上的供体-受体对产生最高的偶极子(气相为16.3 D,水中为29.2 D)。静电势图确认电荷分离,与偶极子方向对齐。总体芳香性指数(MCI, AV1245)随偶极矩的增大而降低,呈中等相关性。将azulene的本征极化与策略取代相结合,使小有机化合物具有超强偶极子,为有机电子学和光电子学相关的高极性π共轭体系提供了基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
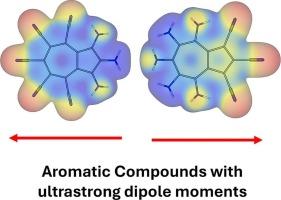
Designing small organic compounds with ultrastrong dipole moments
Azulene's dipole moment was enhanced via electron-donating (–NH₂) and -withdrawing (–CN) group substitution, guided by its reactivity. Derivatives were classified into four groups. DFT calculations show donor-acceptor pairs across rings yield the highest dipoles (up to 16.3 D gas phase, 29.2 D in water). Electrostatic potential maps confirm charge separation, aligning with dipole direction. Global aromaticity (MCI, AV1245 indices) decreases with increasing dipole moment, showing moderate correlation. Combining azulene's intrinsic polarization with strategic substitution enables small organic compounds with ultrastrong dipoles, providing a basis for highly polar π-conjugated systems relevant to organic electronics and optoelectronics.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Physics Letters
化学-物理:原子、分子和化学物理
CiteScore
5.70
自引率
3.60%
发文量
798
审稿时长
33 days
期刊介绍:
Chemical Physics Letters has an open access mirror journal, Chemical Physics Letters: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Chemical Physics Letters publishes brief reports on molecules, interfaces, condensed phases, nanomaterials and nanostructures, polymers, biomolecular systems, and energy conversion and storage.
Criteria for publication are quality, urgency and impact. Further, experimental results reported in the journal have direct relevance for theory, and theoretical developments or non-routine computations relate directly to experiment. Manuscripts must satisfy these criteria and should not be minor extensions of previous work.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


