雷马唑仑通过抑制神经元下垂缓解脑出血结果。
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
脑出血(ICH)是一种严重的脑卒中亚型,具有高发病率和死亡率。脑出血后继发性脑损伤涉及复杂的病理机制,包括神经元凋亡、神经炎症和氧化应激,但有效的治疗干预仍然有限。雷马唑仑是一种超短效镇静麻醉剂,由于其报道的神经保护和抗炎作用,已成为一种有希望的候选者。然而,其在脑出血发病机制中的确切作用尚不清楚。通过小鼠脑出血模型,我们发现雷马唑仑治疗可显著改善神经功能,减少脑水肿,减轻神经元凋亡。鉴于铁下垂在ich诱导的脑损伤中的关键作用,我们进一步研究了它的参与。机制研究表明,雷马唑仑可显著降低脑出血小鼠和hemin处理的HT22细胞血肿周围区域ROS和Fe2+的积累。这些发现强调了雷马唑仑在脑出血中抑制铁下垂和改善认知功能的潜力,为脑出血提供了一个有希望的治疗策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
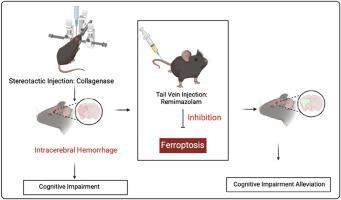
Remimazolam alleviates intracerebral hemorrhage outcomes by suppressing neuronal ferroptosis
Intracerebral hemorrhage (ICH) is a severe stroke subtype associated with high morbidity and mortality. Secondary brain injury after ICH involves complex pathological mechanisms, including neuronal apoptosis, neuroinflammation, and oxidative stress, yet effective therapeutic interventions remain limited. Remimazolam, an ultra-short-acting sedative anesthetic, has emerged as a promising candidate due to its reported neuroprotective and anti-inflammatory effects. However, its precise role in ICH pathogenesis remains unclear. Using a murine ICH model, we found that remimazolam treatment significantly improved neurological function, reduced cerebral edema, and attenuated neuronal apoptosis. Given the critical role of ferroptosis in ICH-induced brain injury, we further investigated its involvement. Mechanistic investigations revealed that remimazolam significantly reduced the accumulation of ROS and Fe2+ in both perihematomal regions of ICH mice and HT22 cells treated with hemin. These findings highlight remimazolam's potential to inhibit ferroptosis and improve cognitive function in ICH, offering a promising therapeutic strategy for ICH.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.00
自引率
0.00%
发文量
572
审稿时长
34 days
期刊介绍:
The European Journal of Pharmacology publishes research papers covering all aspects of experimental pharmacology with focus on the mechanism of action of structurally identified compounds affecting biological systems.
The scope includes:
Behavioural pharmacology
Neuropharmacology and analgesia
Cardiovascular pharmacology
Pulmonary, gastrointestinal and urogenital pharmacology
Endocrine pharmacology
Immunopharmacology and inflammation
Molecular and cellular pharmacology
Regenerative pharmacology
Biologicals and biotherapeutics
Translational pharmacology
Nutriceutical pharmacology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


