癌蛋白snd1富集外泌体通过调节cd47 - sirp α介导的巨噬细胞重编程促进黑色素瘤肺转移。
IF 10.1
1区 医学
Q1 ONCOLOGY
引用次数: 0
摘要
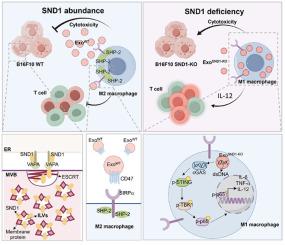
葡萄球菌核酸酶和都铎结构域1 (SND1)是一种在多种肿瘤中高表达的新兴癌蛋白。数据库分析表明,SND1在肿瘤源性外泌体中富集,表明其通过外泌体调节肿瘤微环境(TME)的潜在作用。在这里,我们证明SND1作为一种新的TEXs标记物,通过富集外泌体膜蛋白影响巨噬细胞极化。在小鼠中,SND1富集于黑色素瘤源性外泌体中,促进肺转移,并伴有肿瘤相关巨噬细胞(TAM)浸润增加。相反,snd1缺陷外泌体(ExoSND1-KO)将巨噬细胞极化转向M1表型,创造抗肿瘤免疫微环境并抑制黑色素瘤肺转移。在机制上,SND1促进escrt依赖的CD47分选,从而促进其结合到黑色素瘤来源的外泌体中,并允许它们通过CD47- sirp α轴逃避巨噬细胞介导的吞噬。因此,巨噬细胞不能吞噬TEXs或肿瘤细胞。值得注意的是,缺乏CD47的ExoSND1-KO被巨噬细胞优先吞噬,通过外泌体衍生的dsDNA激活cGAS-STING/TBK1/NF-κB通路触发M1重编程。这一过程导致炎症细胞因子(IL-1β、IL-6、TNF-α)分泌增加和I型细胞介导的免疫激活。我们的研究表明,靶向肿瘤细胞中SND1的富集可能是抑制肿瘤转移的一种有希望的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Oncoprotein SND1-enriched exosomes facilitate melanoma lung metastasis by regulating CD47-SIRPα-mediated macrophage reprogramming
Staphylococcal nuclease and Tudor domain containing 1 (SND1) is an emerging oncoprotein highly expressed in various tumors. Database analyses indicate that SND1 is enriched in tumor-derived exosomes, suggesting its potential role in modulating the tumor microenvironment (TME) via exosomes. Here, we demonstrated that SND1 served as a novel tumor-derived exosome (TEX) marker, influencing macrophage polarization by enriching exosomal membrane proteins. In mice, SND1 enriched in melanoma-derived exosomes promoted lung metastasis, accompanied by increased tumor-associated macrophage (TAM) infiltration. Conversely, SND1-deficient exosomes (ExoSND1−KO) shifted macrophage polarization toward an M1 phenotype, creating an anti-tumor immune microenvironment and inhibiting melanoma lung metastasis. Mechanistically, SND1 promoted ESCRT-dependent CD47 sorting, thereby facilitating its incorporation into melanoma-derived exosomes and allowing them to evade macrophage-mediated phagocytosis through the CD47–SIRPα axis. Consequently, macrophages failed to engulf TEXs or tumor cells. Notably, ExoSND1−KO, lacking CD47, were preferentially phagocytosed by macrophages, triggering M1 reprogramming via exosome-derived dsDNA activation of the cGAS-STING/TBK1/NF-κB pathway. This process led to increased secretion of inflammatory cytokines (IL-1β, IL-6, TNF-α) and activation of type I cell-mediated immunity. Our study suggests that targeting SND1 enrichment in tumor cells could be a promising strategy to inhibit tumor metastasis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cancer letters
医学-肿瘤学
CiteScore
17.70
自引率
2.10%
发文量
427
审稿时长
15 days
期刊介绍:
Cancer Letters is a reputable international journal that serves as a platform for significant and original contributions in cancer research. The journal welcomes both full-length articles and Mini Reviews in the wide-ranging field of basic and translational oncology. Furthermore, it frequently presents Special Issues that shed light on current and topical areas in cancer research.
Cancer Letters is highly interested in various fundamental aspects that can cater to a diverse readership. These areas include the molecular genetics and cell biology of cancer, radiation biology, molecular pathology, hormones and cancer, viral oncology, metastasis, and chemoprevention. The journal actively focuses on experimental therapeutics, particularly the advancement of targeted therapies for personalized cancer medicine, such as metronomic chemotherapy.
By publishing groundbreaking research and promoting advancements in cancer treatments, Cancer Letters aims to actively contribute to the fight against cancer and the improvement of patient outcomes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


