高效大鼠模型中代谢功能障碍相关脂肪变性肝病向肝细胞癌的进展
IF 5.6
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
代谢功能障碍相关脂肪变性肝病(MASLD)已成为一个重要的全球公共卫生问题,是肝细胞癌(HCC)发展的主要危险因素之一;然而,尽管已经提出了一些实验模型,但从MASLD到HCC的时间进展的重述仍然是一个未满足的需求。我们的目标是建立一个有效的MASLD导致HCC的模型,命名为MACER (MASLD- cancer)模型。体重100-120 g的6周龄雄性Fischer 344大鼠被喂食含有高水平脂肪、蔗糖和胆固醇的致肝性(HPG)饮食,并与低剂量CCl4和二乙基亚硝胺(DEN)结合。评估肝骨变性、炎症、纤维化和HCC标志物。证据显示,在暴露于肝毒素后的第三周,MACER模型诱导了脂质代谢的改变,促进了肝骨疏松症,增加了肝损伤、炎症和细胞衰老。此外,该模型在7周时表达增殖标志物Ki-67,并在13 周后诱导胶原沉积和表达HCC标志物,如GGT和GSTP1。此外,即使在去除肝毒素后,我们的模型也进展到晚期HCC阶段。MACER模型是一种新颖而有用的工具,用于研究按时间顺序排列的MASLD对HCC进展的影响,以及评估化学预防药物的疗效。本文章由计算机程序翻译,如有差异,请以英文原文为准。
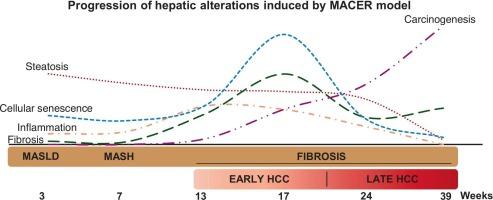
The progression of metabolic dysfunction-associated steatotic liver disease toward hepatocellular carcinoma in an efficient rat model
Metabolic dysfunction-associated steatotic liver disease (MASLD) has emerged as a critical worldwide public health issue and is among the main risk factors for hepatocellular carcinoma (HCC) development; however, although some experimental models have been proposed, the recapitulation of the chronological progression from MASLD to HCC remains an unmet need. We aimed to establish an efficient MASLD model leading to HCC, named the MACER (MASLD-CANCER) model. Six-week-old male Fischer 344 rats, weighing 100–120 g, were fed a hepatopathogenic (HPG) diet containing high levels of fat, sucrose, and cholesterol in combination with low doses of CCl4 and diethylnitrosamine (DEN). Hepatosteatosis, inflammation, fibrosis, and HCC markers were assessed. The evidence revealed that the MACER model induced an altered lipid metabolism profile, promoted hepatosteatosis, and increased liver injury, inflammation, and cellular senescence beginning the third week after exposure to hepatotoxins. In addition, this model showed expression of the proliferation marker Ki-67 at seven weeks and induction of collagen deposition and expression of HCC markers, such as GGT and GSTP1, from 13 weeks onward. Moreover, our model progressed to advanced HCC stages even after removing hepatotoxins. The MACER model is a novel and useful tool for investigating chronological MASLD toward HCC progression and for assessing the efficacy of chemopreventive agents.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


