3-氨基吡唑衍生物的光催化氟烷基化反应
IF 0.9
4区 化学
Q4 CHEMISTRY, ORGANIC
引用次数: 0
摘要
介绍了在钌光催化剂存在下,3-氨基吡唑衍生物与碘化五氟乙基在蓝光照射下的反应。在4号或5号位置有取代基的3-氨基吡唑产生相应的氟烷基化产物。在3-氨基吡唑的N-Boc衍生物中,自由基氟烷基化的选择性取决于N-Boc吡唑的结构。本文章由计算机程序翻译,如有差异,请以英文原文为准。
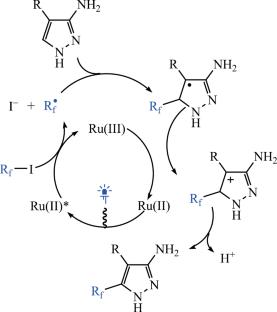
Photocatalytic Fluoroalkylation of Derivatives of 3-Aminopyrazole
The reaction of 3-aminopyrazole derivatives with pentafluoroethyl iodide in the presence of a ruthenium photocatalyst under blue light irradiation is described. 3-Aminopyrazoles bearing a substituent at position 4 or 5 yielded the corresponding fluoroalkylation products. In the case of N-Boc derivatives of 3-aminopyrazole, the selectivity of radical fluoroalkylation depended on the structure of the N-Boc pyrazole.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
1.40
自引率
25.00%
发文量
139
审稿时长
3-6 weeks
期刊介绍:
Russian Journal of Organic Chemistry is an international peer reviewed journal that covers all aspects of modern organic chemistry including organic synthesis, theoretical organic chemistry, structure and mechanism, and the application of organometallic compounds in organic synthesis.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


