铅-石墨复合电极上CO2电催化转化为甲酸盐的研究
摘要
在减少二氧化碳排放和减缓气候变化的不同途径中,通过电化学还原将二氧化碳回收为增值产品是有前途的。本研究强调了在h型电池中使用铅-石墨复合电极将CO2转化为甲酸。可变wt的不同复合电极 % of Pb and graphite were investigated. Electrode characterization using energy dispersive X-ray and Scanning electron microscope provided a porous surface with partially flaky crack morphology and a homogeneous distribution of Pb in the graphite matrix. Absorption of CO2 by 0.1 M KHCO3 at 25°C and 1 atm provided a value of 1.434 g/L (30.66 mol/L). Adsorption of the dissolved CO2 by 50 wt % Pb electrodes demonstrated a saturation capacity of 590 mg/g. Cyclic voltammetry showed a distinct reduction peak of CO2 at –0.62 V (vs. Ag/AgCl). This peak has increased with an increased amount of absorbed CO2 in 0.1 M KHCO3. Linear Sweep Voltammetry provided an irreversible conversion of CO2 to formate with a peak current of 32.5 mA at a scan rate of 0.1 V/s and 1 M KCO3. Electrode kinetic analysis proved a Butler–Volmer reduction constant of \(\beta = 0.62\) at 298 K, leading to a differential change in reaction constant with the potential 77.89 \({{{\text{V}}}^{{ - 1}}}{{\;}}{{{\text{s}}}^{{ - 1}}}\). The corresponding current efficiency was varied with a variation of KHCO3 concentration to yield a value of 96% obtained using 1 M KHCO3 and 25°C. Therefore, the composite Pb-graphite electrode demonstrated high surface area, minimum mass transfer, and diffusion resistance of the dissolved CO2 to the electrode surface.
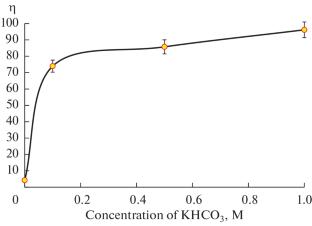
Among the different approaches for CO2 emanation alleviation and climate change mitigation, recycling CO2 into added-value products through electrochemical reduction is promising. This study highlights the conversion of CO2 to formate using a composite electrode of Pb-graphite in an H-type cell. Different composite electrodes with variable wt % of Pb and graphite were investigated. Electrode characterization using energy dispersive X-ray and Scanning electron microscope provided a porous surface with partially flaky crack morphology and a homogeneous distribution of Pb in the graphite matrix. Absorption of CO2 by 0.1 M KHCO3 at 25°C and 1 atm provided a value of 1.434 g/L (30.66 mol/L). Adsorption of the dissolved CO2 by 50 wt % Pb electrodes demonstrated a saturation capacity of 590 mg/g. Cyclic voltammetry showed a distinct reduction peak of CO2 at –0.62 V (vs. Ag/AgCl). This peak has increased with an increased amount of absorbed CO2 in 0.1 M KHCO3. Linear Sweep Voltammetry provided an irreversible conversion of CO2 to formate with a peak current of 32.5 mA at a scan rate of 0.1 V/s and 1 M KCO3. Electrode kinetic analysis proved a Butler–Volmer reduction constant of \(\beta = 0.62\) at 298 K, leading to a differential change in reaction constant with the potential 77.89 \({{{\text{V}}}^{{ - 1}}}{{\;}}{{{\text{s}}}^{{ - 1}}}\). The corresponding current efficiency was varied with a variation of KHCO3 concentration to yield a value of 96% obtained using 1 M KHCO3 and 25°C. Therefore, the composite Pb-graphite electrode demonstrated high surface area, minimum mass transfer, and diffusion resistance of the dissolved CO2 to the electrode surface.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


