MPO3(M2WO4) -V2O5 (M = Na, K)熔体电导率的实验研究
IF 0.8
4区 化学
Q4 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
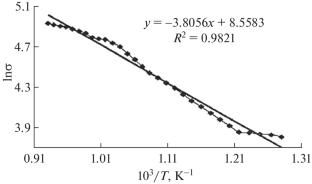
研究了KPO3-V2O5、NaPO3-V2O5、Na2WO4-V2O5和K2WO4-V2O5二元共晶氧化物盐复合材料的电导率。测定了电导率对数和比电导率随温度倒数的变化规律。结果表明,38 mol %的NaPO3-62 mol %的V2O5氧化物盐复合材料具有最高的电导率,有望用作难熔金属的电解液和化学电流源。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Experimental Study of Electric Conductivity of MPO3(M2WO4)–V2O5 (M = Na, K) Melts
The electric conductivity of binary eutectic oxide–salt composites KPO3–V2O5, NaPO3–V2O5, Na2WO4–V2O5, and K2WO4–V2O5 has been studied. The behavior of the dependence of the conductivity logarithm and specific conductivity on the reciprocal temperature was determined. It was established that the 38 mol % NaPO3–62 mol % V2O5 oxide–salt composite has the highest electric conductivity and is promising for use as an electrolyte for obtaining refractory metals and in chemical current sources.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
1.20
自引率
14.30%
发文量
376
审稿时长
5.1 months
期刊介绍:
Russian Journal of Physical Chemistry A. Focus on Chemistry (Zhurnal Fizicheskoi Khimii), founded in 1930, offers a comprehensive review of theoretical and experimental research from the Russian Academy of Sciences, leading research and academic centers from Russia and from all over the world.
Articles are devoted to chemical thermodynamics and thermochemistry, biophysical chemistry, photochemistry and magnetochemistry, materials structure, quantum chemistry, physical chemistry of nanomaterials and solutions, surface phenomena and adsorption, and methods and techniques of physicochemical studies.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


