la3ta7的热容和热力学性质
IF 0.8
4区 化学
Q4 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
采用松弛法、绝热法和差示扫描量热法在0 ~ 1860 K的温度范围内测定了网状结构型钽酸镧La3TaO7的摩尔热容;热力学性质:由热容的光滑值计算熵和焓增量;估算了高温区二元氧化物生成钽酸镧的吉布斯能;la3ta7具有较高的稳定性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
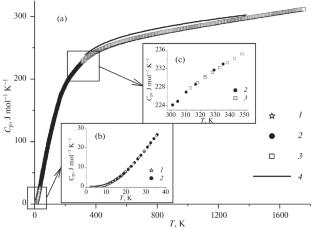
Heat Capacity and Thermodynamic Properties of La3TaO7
The molar heat capacity of lanthanum tantalate La3TaO7 of the weberite structural type was measured by the relaxation, adiabatic, and differential scanning calorimetry methods in the temperature range of 0–1860 K; the thermodynamic properties: entropy and enthalpy increment were calculated from the smoothed values of heat capacity; the Gibbs energy of formation of lanthanum tantalate from binary oxides in the high-temperature region was estimated; and La3TaO7 was shown to have high stability.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
1.20
自引率
14.30%
发文量
376
审稿时长
5.1 months
期刊介绍:
Russian Journal of Physical Chemistry A. Focus on Chemistry (Zhurnal Fizicheskoi Khimii), founded in 1930, offers a comprehensive review of theoretical and experimental research from the Russian Academy of Sciences, leading research and academic centers from Russia and from all over the world.
Articles are devoted to chemical thermodynamics and thermochemistry, biophysical chemistry, photochemistry and magnetochemistry, materials structure, quantum chemistry, physical chemistry of nanomaterials and solutions, surface phenomena and adsorption, and methods and techniques of physicochemical studies.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


