1,5-二氢- 2h -吡咯-2- 1系列叠氮化物晶体中有限固溶体的形成作为手性判别的局部损失
IF 1.4
4区 化学
Q4 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
利用5-甲氧基-2(5H)-呋喃酮- 4-叠氮酮衍生物与2-氨基乙醇相互作用,合成了新的含氮杂环4-叠氮酮-3-溴-5-羟基-1-(2-羟乙基)-1,5-二氢- 2h -吡咯-2- 1。XRD证实该化合物结晶为含有等量的异手性和同手性氢键二聚体的有限固溶体。量子化学方法表明,这两种二聚体都是稳定的,并且表现出相似的内相互作用能量。讨论了局部手性判别损失的原因。本文章由计算机程序翻译,如有差异,请以英文原文为准。
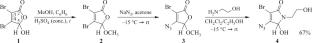
Formation of a Limited Solid Solution in a Crystalline Azide of the 1,5-Dihydro-2H-Pyrrol-2-One Series as a Local Loss of Chiral Discrimination
A new nitrogen-containing heterocycle, 4-azido-3-bromine-5-hydroxy-1-(2-hydroxyethyl)-1,5-dihydro-2H-pyrrol-2-one, is prepared by the interaction of a 5-methoxy-2(5H)-furanone 4-azido derivative with 2-aminoethanol. It is established by XRD that this compound crystallizes as a limited solid solution containing equal amounts of hetero- and homochiral H-bonded dimers. It is shown by quantum chemical methods that both dimers are stable and exhibit similar energies of intradimer interactions. The reasons of local chiral discrimination loss are discussed.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Structural Chemistry
化学-无机化学与核化学
CiteScore
1.60
自引率
12.50%
发文量
142
审稿时长
8.3 months
期刊介绍:
Journal is an interdisciplinary publication covering all aspects of structural chemistry, including the theory of molecular structure and chemical bond; the use of physical methods to study the electronic and spatial structure of chemical species; structural features of liquids, solutions, surfaces, supramolecular systems, nano- and solid materials; and the crystal structure of solids.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


