Glypican 1机械传感介导静液性肺水肿中eNOS解偶联
IF 11.9
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
静液性肺水肿是由肺毛细血管压力急性升高引起的危及生命的疾病。静液性肺水肿发生的分子机制尚不清楚。肺内皮糖萼是调节肺内皮通透性的机械敏感信号层。在糖萼内,膜结合的硫酸肝素蛋白聚糖(HSPGs)被认为是机械传感器。本文中,我们研究了膜结合的HSPG糖化蛋白1是否是肺血管中的机械传感器及其在静液性肺水肿进展中的作用。通过分离的灌注肺系统,我们发现glypican 1敲除小鼠(Gpc1−/−)免受压力性肺水肿的影响,这一表型与70 KDa葡聚糖转运受损和活性氧(ROS)产生减少有关。利用野生型(WT)小鼠肺内皮细胞(MLEC)和人肺微血管内皮细胞(HLMEC),我们发现高压诱导蛋白激酶c - α (PKCα)在Y657位点的激活,使内皮型一氧化氮合酶(eNOS)在T495位点磷酸化。这与通过enos依赖途径增加ROS产生有关。乙基硫脲(ETU)或N5-(1-亚氨基乙基)-l-鸟氨酸(L-NIO)抑制eNOS可减轻高压对ROS生成、肺水肿和屏障稳定性的影响。在高压条件下的Gpc1−/−MLEC中,这种病理性信号轴不被激活。值得注意的是,Glypican 1缺失的细胞显示PKCα在T638位点的磷酸化增加,这是一个与PKCα稳定性和失活相关的位点。在Gpc1−/−MLEC中观察到的保护性信号机制在glypican 1沉默的HLMEC中得到复制,支持glypican 1在跨物种屏障功能中的保守作用。总之,我们发现glypican 1是肺血管中的一种机械传感器,通过氧化还原敏感途径介导高压对屏障功能的影响。这可能对人体静压性肺水肿的进展很重要。针对glypican 1的治疗可能是治疗静液性肺水肿的新策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
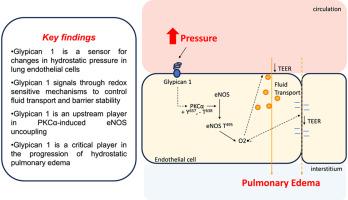
Glypican 1 mechanosensing mediates eNOS uncoupling during hydrostatic pulmonary edema
Hydrostatic pulmonary edema is a life-threatening condition caused by an acute increase in pulmonary capillary pressure. The molecular mechanisms whereby hydrostatic pulmonary edema develops are unresolved. The pulmonary endothelial glycocalyx is a mechano-sensitive signaling layer known to regulate lung endothelial permeability. Within the glycocalyx, membrane-bound heparan sulfate proteoglycans (HSPGs) are putative mechano-sensors. Herein, we investigated if the membrane-bound HSPG glypican 1 is a mechanosensor in the lung vasculature and its role in hydrostatic pulmonary edema progression. Using an isolated perfused lung system, we showed that glypican 1 knockout mice (Gpc1−/−) are protected from pressure-induced lung edema, a phenotype associated with impaired 70 KDa dextran transport and decreased reactive oxygen species (ROS) production. Using wild-type (WT) mouse lung endothelial cells (MLEC) and human lung microvascular endothelial cells (HLMEC), we show that high pressure induces the activation of Protein Kinase C-alpha (PKCα) at Y657, which phosphorylates endothelial nitric oxide synthase (eNOS) at T495. This is associated with increased ROS production by eNOS-dependent pathways. The inhibition of eNOS with ethyl thiourea (ETU) or N5-(1-iminoethyl)-l-ornithine (L-NIO) mitigates the effects of high pressure on ROS production, lung edema, and barrier stability. This pathologic signaling axis is not activated in Gpc1−/− MLEC exposed to high-pressure conditions. Notably, cells deficient in Glypican 1 show increased phosphorylation of PKCα at T638, a site associated with PKCα stability and inactivation. The protective signaling mechanisms observed in Gpc1−/− MLEC are replicated in HLMEC silenced for glypican 1, supporting a conserved role for glypican 1 in barrier function across species. In conclusion, we show that glypican 1 is a mechanosensor in the lung vasculature that mediates the effects of high pressure on barrier function by redox-sensitive pathways. This may be important for the progression of hydrostatic pulmonary edema in humans. Therapies targeting glypican 1 may be novel strategies to treat hydrostatic pulmonary edema.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Redox Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-
CiteScore
19.90
自引率
3.50%
发文量
318
审稿时长
25 days
期刊介绍:
Redox Biology is the official journal of the Society for Redox Biology and Medicine and the Society for Free Radical Research-Europe. It is also affiliated with the International Society for Free Radical Research (SFRRI). This journal serves as a platform for publishing pioneering research, innovative methods, and comprehensive review articles in the field of redox biology, encompassing both health and disease.
Redox Biology welcomes various forms of contributions, including research articles (short or full communications), methods, mini-reviews, and commentaries. Through its diverse range of published content, Redox Biology aims to foster advancements and insights in the understanding of redox biology and its implications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


