天然支架,现代策略:香豆素杂交体作为潜在的碳水化合物消化酶抑制剂——药物化学探索十年(2015-2025)
IF 3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
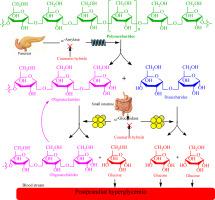
在过去的十年(2015-2025)中,香豆素及其杂交衍生物已成为寻找有效的糖消化酶抑制剂的有前景的支架,特别是α-淀粉酶(AMY)和α-葡萄糖苷酶(AG),它们是控制2型糖尿病患者餐后高血糖的关键药理靶点。本文综述了以香豆素为基础的杂合体的药物化学研究成果,重点介绍了它们的开发策略、合成、酶抑制潜力和构效关系(SAR)。各种药效偶联物,包括噻唑、噻唑烷二酮、三唑、查尔酮、isatin、磺胺、恶二唑、腙和肉桂酸,已被有效地连接到香豆素核上,以增强酶抑制电位。对接分析表明,与标准药物阿卡波糖相比,几种衍生物具有更好的酶抑制作用,具有良好的体外和体内特征,低细胞毒性和增强的结合相互作用。研究结果表明,取代模式、电子效应和连接物修饰显著影响生物活性。这些发现强调了香豆素作为铅支架的多功能性,并为合理设计针对这两种碳水化合物消化酶的下一代铅分子提供了坚实的基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Natural scaffold, modern strategy: Coumarin hybrids as potential carbohydrate-digesting enzyme inhibitors-A decade of medicinal chemistry exploration (2015–2025)
Over the past decade (2015–2025), coumarin and its hybrid derivatives have emerged as promising scaffolds in the search for potent inhibitors of carbohydrate-digesting enzymes, particularly α-amylase (AMY) and α-glucosidase (AG), which are key pharmacological targets in controlling postprandial hyperglycemia in type-2 diabetic patients. This review compiles and critically evaluates the medicinal chemistry efforts focused on coumarin-based hybrids, highlighting their development strategies, synthesis, enzyme inhibitory potential, and structure–activity relationships (SAR). Various pharmacophoric conjugates, including thiazole, thiazolidinedione, triazole, chalcone, isatin, sulfonamide, oxadiazole, hydrazone, and cinnamic acid, have been effectively linked to the coumarin nucleus to enhance enzyme inhibitory potential. Several derivatives have exhibited superior enzyme inhibition compared to the standard drug acarbose, with favorable in vitro and in vivo profiles, low cytotoxicity, and enhanced binding interactions as supported by docking analysis. The SAR insights reveal that substitution patterns, electronic effects, and linker modifications significantly influence biological activity. The findings underscore the versatility of coumarin as a lead scaffold and deliver a robust foundation for the rational design of next-generation lead molecules targeting the two carbohydrate-digesting enzymes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


