新型THR-β激动剂的结构设计及分子动力学模拟活化机制研究
IF 3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
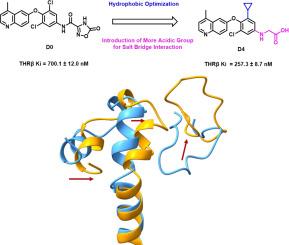
甲状腺激素受体β (THR-β)在调节脂质代谢、脂肪性肝炎和肝纤维化中起关键作用。瑞司替龙的批准证明了THR-β激动剂在治疗代谢相关脂肪性肝炎(MASH)方面的治疗潜力。因此,研究THR-β的活化机制对了解其作用方式和进一步研究具有重要意义。在这项研究中,我们报道了一种新设计的THR-β激动剂D4,它具有优异的体外活性,Ki值为257.3 nM,为探索受体激活提供了有价值的工具。为了深入了解机制,我们对三种体系进行了500 ns的分子动力学(MD)模拟:THR-β与激动剂D4结合,THR-β与已知拮抗剂6结合,以及载子THR-β(无配体)。对这些体系的结构稳定性和活化机理进行了详细的分析。我们的发现提示了一种新的激活机制,其中由环- h11 - h12介导的动态盐桥中继稳定了受体的活性状态。此外,拮抗剂与Arg438碳链形成的关键疏水相互作用是配体从激动剂转变为拮抗剂的关键。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Structure-based design of novel THR-β agonists and mechanism of activation research by molecular dynamics simulations
Thyroid hormone receptor β (THR-β) plays a crucial role in regulating lipid metabolism, steatohepatitis, and liver fibrosis. The approval of Resmetirom has demonstrated the therapeutic potential of THR-β agonism in treating metabolic-associated steatohepatitis (MASH). Therefore, investigating the mechanism of THR-β activation is essential for understanding its mode of action and further research. In this study, we report a newly designed THR-β agonist, D4, which exhibits superior in vitro activity, with a Ki value of 257.3 nM, providing a valuable tool for exploring receptor activation. To gain mechanistic insights, we conducted 500 ns molecular dynamics (MD) simulations on three systems: THR-β bound to agonist D4, THR-β bound to a known antagonist 6, and apo THR-β (ligand-free). A detailed analysis of the structural stability and activation mechanism of these systems was performed. Our findings suggest a novel activation mechanism in which a dynamic salt bridge relay, mediated by Loop-H11-H12, stabilizes the active state of the receptor. In addition, key hydrophobic interaction formed by antagonist and carbon chain of Arg438 was found to be crucial for ligands to switch from agonist to antagonist.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


