左旋四氢巴马汀靶向非小细胞肺癌hTERT g -四重体的结构和功能研究
IF 3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
人类端粒酶逆转录酶(hTERT)启动子中的g -四重体结构最近成为癌症治疗的一个有希望的靶点。然而,选择性配体的缺乏严重制约了其开发和应用。本研究利用荧光光谱、紫外可见吸收、圆二色性(CD)、CD熔化试验、电泳迁移位移试验(EMSA)、分子对接以及细胞和体内模型等方法,将左旋四氢巴马汀(l-THP)及其两个代谢衍生物(M1和M2)鉴定为hTERT g -四联体的新型配体。我们的研究结果表明,l-THP、M1和M2对hTERT g -四重体具有潜在的结合亲和力和选择性。荧光突变分析显示,这些化合物优先与第一和第二g -束(5 ' -3 ')之间的连接相互作用,特别是识别21位的胞嘧啶。此外,这些配体显著抑制hTERT mRNA的表达(对照组为1.00,l-THP为0.43±0.02,M1为0.19±0.01,M2为0.33±0.03),并在A549细胞和肺癌异种移植模型中显示出强大的抗肿瘤活性。总的来说,这些发现确立了l-THP及其衍生物作为开发非小细胞肺癌化疗药物的有希望的先导化合物,并为有效的抗肿瘤活性提供了合理的机制见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
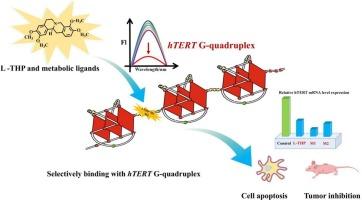
Structural and functional insights into targeting hTERT G-quadruplex by levo-Tetrahydropalmatine in the non-small cell lung cancer
The G-quadruplex structure in the human telomerase reverse transcriptase (hTERT) promoter has recently emerged as a promising therapeutic target in cancer. However, the development and application are strongly restricted by the lack of selective ligands. Herein, levo-tetrahydropalmatine (l-THP) and its two metabolic derivatives (M1 and M2) were identified as novel ligands of the hTERT G-quadruplex using a combination of fluorescence spectroscopy, UV–visible absorption, circular dichroism (CD), CD-melting assays, electrophoretic mobility shift assays (EMSA), molecular docking, as well as cellular and in vivo models. Our results demonstrate that l-THP, M1, and M2 exhibit potential binding affinity and selectivity toward the hTERT G-quadruplex. Fluorescence mutation assays revealed that these compounds preferentially interact with the junction between the first and second G-tracts (5′-3′), particularly recognizing the cytosine at position 21. Moreover, these ligands significantly suppressed the hTERT mRNA expression (1.00 for control, 0.43 ± 0.02 for l-THP, 0.19 ± 0.01 for M1, 0.33 ± 0.03 for M2) and showed the potent antitumor activity in A549 cells and lung cancer xenograft model. Collectively, these findings establish l-THP and its derivatives as the promising lead compounds for the development of chemotherapeutics against non-small cell lung cancer as well as provide the plausible mechanism insights into potent antitumor activity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


