不同益生菌发酵酪蛋白水解产物中ace抑制肽的鉴定与筛选
IF 3.4
3区 农林科学
Q2 FOOD SCIENCE & TECHNOLOGY
引用次数: 0
摘要
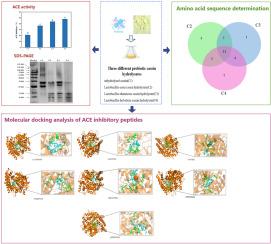
本研究旨在探讨不同益生菌发酵酪蛋白对促进血管紧张素转换酶抑制(ACE-I)肽释放的影响。分析比较了不同益生菌对蛋白质水解程度、ACE-I活性和酪蛋白氨基酸序列的影响。利用分子对接分析了肽与ACE分子的结合位点。与其他益生菌相比,helveticus乳杆菌酪蛋白水解物(C4)的ACE-I活性最高(48.12%),且大部分肽段小于1.6 kDa。此外,52.17 - 86.67%的ACE-I肽来自β-酪蛋白(β-CN)。由helveticus乳杆菌水解酪蛋白产生的PYVRYL可以在多个位点与ACE结合,是一种高效的ACE- i肽。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Identification and in-silico screening of ACE-inhibitory peptides from casein hydrolysate via fermentation with different probiotics
This study aims to explore the effect of using different probiotics to ferment casein in order to promote the release of angiotensin-converting enzyme inhibition (ACE-I) peptides. The effects of different probiotics on the degree of protein hydrolysis, ACE-I activity, and the amino acid sequence of casein were analyzed and compared. The binding sites of peptides to the ACE molecule were analyzed using molecular docking. Compared with other probiotics, the ACE-I activity of Lactobacillus helveticus casein hydrolysate (C4) was the highest (48.12 %), and most of the peptides were smaller than 1.6 kDa. Additionally, 52.17–86.67 % of the ACE-I peptides came from β-casein (β-CN). The PYVRYL produced by Lactobacillus helveticus hydrolyzing casein can bind to ACE at multiple sites and is an efficient ACE-I peptide.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

International Dairy Journal
工程技术-食品科技
CiteScore
6.50
自引率
9.70%
发文量
200
审稿时长
49 days
期刊介绍:
The International Dairy Journal publishes significant advancements in dairy science and technology in the form of research articles and critical reviews that are of relevance to the broader international dairy community. Within this scope, research on the science and technology of milk and dairy products and the nutritional and health aspects of dairy foods are included; the journal pays particular attention to applied research and its interface with the dairy industry.
The journal''s coverage includes the following, where directly applicable to dairy science and technology:
• Chemistry and physico-chemical properties of milk constituents
• Microbiology, food safety, enzymology, biotechnology
• Processing and engineering
• Emulsion science, food structure, and texture
• Raw material quality and effect on relevant products
• Flavour and off-flavour development
• Technological functionality and applications of dairy ingredients
• Sensory and consumer sciences
• Nutrition and substantiation of human health implications of milk components or dairy products
International Dairy Journal does not publish papers related to milk production, animal health and other aspects of on-farm milk production unless there is a clear relationship to dairy technology, human health or final product quality.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


