巴西东北部Mirandiba沉积盆地地下水资源评价
IF 3.4
3区 地球科学
Q1 GEOCHEMISTRY & GEOPHYSICS
引用次数: 0
摘要
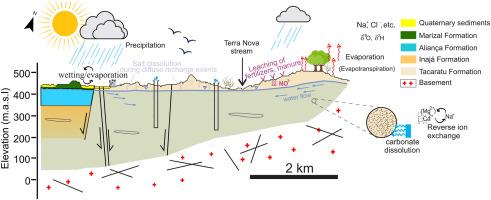
在半干旱地区,地下水是一种重要的水资源,受到盐渍化和污染的威胁。研究了封闭盆地的地球物理、理化和同位素组成(δ18O和δ2H),确定了封闭盆地的一般几何特征,确定了控制地下水地球化学和水质的成因和主要演化过程,为有效的水资源管理提供了可靠的信息。在巴西东北部半干旱气候的作用下,Mirandiba盆地以无约束为主,缺乏蒸发(岩盐)沉积,但氯离子含量较高。地球物理揭示了一个厚度小于400 m的浅盆,基底形态为半地堑构造。地下水样品分为3组:1)簇1样品对应矿化程度较高的混合阳离子- cl至Na-Cl微酸性水,2)簇2样品对应矿化程度较低的Na-Cl酸性水,3)簇3样品对应中性混合阳离子-混合阴离子水。δ18O和δ2H的同位素特征分别为- 4.95 ~ - 3.16‰和- 29.74 ~ - 17.50‰,表明地下水来源于雨水,部分样品显示有蒸发作用。综合技术分析表明,控制地下水化学演化的主要因素有:1)湿润/蒸发引起的千年循环盐(主要是岩盐)的积累和补给期的后期溶解淋滤;2)岩石风化;3)反向离子交换;4)人为硝酸盐污染。约90%的地下水样本的物理化学参数符合世卫组织的指导方针,约92%的样本属于饮用水质良好和优良的类别。同样,基于灌溉的指数显示了灌溉的总体适宜性。本研究采用的方法有助于识别地下水的主要过程(自然过程和人为过程),并用于建立概念水文地质模型,以建立干旱至半干旱地区含水层的有效管理策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Groundwater resources assessment in the Mirandiba sedimentary Basin at NE Brazil
In semi-arid regions, groundwater is a vital water resource threatened by salinization and contamination. Geophysical and the physicochemical and isotopic compositions (δ18O and δ2H) are investigated to determine the general geometrical characteristics of a closed basin and identify the origin and main evolutionary processes controlling the groundwater geochemistry and quality to provide reliable information for effective water management. The studied dominantly unconfined Mirandiba Basin lacks evaporitic (halite) deposits but contains comparatively higher chloride concentration, under the role of the semi-arid climate of NE Brazil. Geophysics unravels a shallow basin with a thickness lower than 400 m and a basement morphology suggesting a half-graben structure. Groundwater samples were classified into three groups: 1) cluster 1 samples correspond to more mineralized mixed cations–Cl to Na–Cl slightly acid waters, 2) cluster 2 samples correspond to less mineralized Na–Cl acid waters, and 3) cluster 3 samples correspond to neutral mixed cations-mixed anions waters. The isotopic signatures range from −4.95 to −3.16 ‰ and – 29.74 to −17.50 ‰ for δ18O and δ2H, respectively, indicating that groundwater is derived from rainwater with some samples suggesting evaporation. The integrated techniques have shown that the main factors controlling groundwater chemistry evolution are related to 1) wetting/evaporation that induced millennial cyclic salt (mainly halite) accumulation and later dissolution and leaching during recharge periods, 2) rock weathering, 3) reverse ion exchange, and 4) anthropogenic nitrate contamination. The physicochemical parameters of ∼90 % of groundwater samples are within the WHO guidelines, and ∼92 % within the good and excellent water quality category for drinking purposes. Similarly, irrigation ion-based indices showed overall suitability for irrigation. The approach followed in this study is useful for recognize the main processes (natural and anthropogenic) operating in groundwater, and was used to build a conceptual hydrogeologic model, for establishing effective management strategies in aquifers in arid to semi-arid areas.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Applied Geochemistry
地学-地球化学与地球物理
CiteScore
6.10
自引率
8.80%
发文量
272
审稿时长
65 days
期刊介绍:
Applied Geochemistry is an international journal devoted to publication of original research papers, rapid research communications and selected review papers in geochemistry and urban geochemistry which have some practical application to an aspect of human endeavour, such as the preservation of the environment, health, waste disposal and the search for resources. Papers on applications of inorganic, organic and isotope geochemistry and geochemical processes are therefore welcome provided they meet the main criterion. Spatial and temporal monitoring case studies are only of interest to our international readership if they present new ideas of broad application.
Topics covered include: (1) Environmental geochemistry (including natural and anthropogenic aspects, and protection and remediation strategies); (2) Hydrogeochemistry (surface and groundwater); (3) Medical (urban) geochemistry; (4) The search for energy resources (in particular unconventional oil and gas or emerging metal resources); (5) Energy exploitation (in particular geothermal energy and CCS); (6) Upgrading of energy and mineral resources where there is a direct geochemical application; and (7) Waste disposal, including nuclear waste disposal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


