新型萘普生-非那西丁-三唑类抗炎药的合成及生物学评价
IF 4.7
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
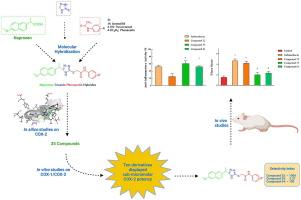
传统的非甾体抗炎药(NSAIDs)抑制COX-1和COX-2亚型,选择性有限,经常导致胃肠道(GI)副作用。优先抑制COX-2而不是COX-1的化合物被认为具有较低的溃疡发生风险。为了开发更安全、更具成本效益的抗炎药物,我们设计了将萘普生和非那西丁与1,2,4-三唑-5-硫酮结合的杂交分子。通过IR、1H/13C NMR和HR-ESI-MS对合成化合物的结构进行了确证。其中,10种化合物对COX-2的抑制作用IC₅₀≤0.56 μM, 5种化合物具有高选择性(SI≥100)。化合物46 (IC₅₀= 0.16 μM, SI = 2.36)、55 (0.19 μM, SI = 2.27)和59 (0.21 μM, SI = 1.65)的效价与塞来昔布相当。通过卡拉胶诱导的足跖水肿模型和瑞士小鼠的急性胃毒性进一步在体内评估三种选定的化合物(n = 6; Ethics No. 2014 /11 08)。口服化合物55和64 (20mg /kg)分别减少了61%和52%的足跖水肿,与吲哚美辛(52%)相似,没有引起明显的胃损伤(损伤评分为2对5)。活性化合物的分子对接研究显示,在COX-2侧袋内与His90和Arg513形成稳定的氢键,与选择性COX-2抑制剂的结合模式相似。通过SwissADME和PreADMET进行的计算机ADME评估表明,这些化合物符合Lipinski的五项标准,具有最佳的极性表面积和Caco-2渗透性,并且显示出最小的局部胃刺激风险,表明有希望的口服生物利用度。总的来说,1,2,4-三唑-硫酮-萘普生/非那西丁杂交体是开发gi安全非甾体抗炎药的有希望的主要候选药物。特别是化合物55和64,值得进一步优化和长期安全性评价。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Synthesis and biological evaluation of novel naproxen–phenacetin triazole hybrids as promising anti-inflammatory agents with enhanced gastrointestinal tolerability
Traditional nonsteroidal anti-inflammatory drugs (NSAIDs) inhibit both COX-1 and COX-2 isoforms with limited selectivity, often resulting in gastrointestinal (GI) side effects. Compounds that preferentially inhibit COX-2 over COX-1 are believed to pose a lower ulcerogenic risk. To develop GI-safer and cost-effective anti-inflammatory agents, we designed hybrid molecules by combining naproxen and phenacetin with a 1,2,4-triazole-5-thione. The structures of synthesized compounds were confirmed via IR, 1H/13C NMR, and HR-ESI-MS analyses. Among the compounds, ten inhibited COX-2 with IC₅₀ ≤ 0.56 μM, and five exhibited high selectivity (SI ≥ 100). Compounds 46 (IC₅₀ = 0.16 μM, SI = 2.36), 55 (0.19 μM, SI = 2.27), and 59 (0.21 μM, SI = 1.65) demonstrated potency comparable to celecoxib. Three selected compounds were further evaluated in vivo using a carrageenan-induced paw oedema model and for acute gastric toxicity in Swiss mouse (n = 6; Ethics No. 2024/11 08). Orally administered compounds 55 and 64 (20 mg/kg) reduced paw oedema by 61 % and 52 %, respectively, similar to indomethacin (52 %), without causing visible gastric lesions (lesion score ∼ 2 vs. 5 for indomethacin). Molecular docking studies of the active compounds revealed the formation of stable hydrogen bonds with His90 and Arg513 within the COX-2 side pocket, similar to the binding pattern observed in selective COX-2 inhibitors. In silico ADME assessments via SwissADME and PreADMET indicated that these compounds meet Lipinski's rule of five criteria, possess optimal polar surface area and Caco-2 permeability, and show minimal risk for local gastric irritation, suggesting promising oral bioavailability. Overall, the 1,2,4-triazole-thione–naproxen/phenacetin hybrids represent promising lead candidates for the development of GI-safer NSAIDs. Compounds 55 and 64, in particular, merit further optimization and long-term safety evaluation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic Chemistry
生物-生化与分子生物学
CiteScore
9.70
自引率
3.90%
发文量
679
审稿时长
31 days
期刊介绍:
Bioorganic Chemistry publishes research that addresses biological questions at the molecular level, using organic chemistry and principles of physical organic chemistry. The scope of the journal covers a range of topics at the organic chemistry-biology interface, including: enzyme catalysis, biotransformation and enzyme inhibition; nucleic acids chemistry; medicinal chemistry; natural product chemistry, natural product synthesis and natural product biosynthesis; antimicrobial agents; lipid and peptide chemistry; biophysical chemistry; biological probes; bio-orthogonal chemistry and biomimetic chemistry.
For manuscripts dealing with synthetic bioactive compounds, the Journal requires that the molecular target of the compounds described must be known, and must be demonstrated experimentally in the manuscript. For studies involving natural products, if the molecular target is unknown, some data beyond simple cell-based toxicity studies to provide insight into the mechanism of action is required. Studies supported by molecular docking are welcome, but must be supported by experimental data. The Journal does not consider manuscripts that are purely theoretical or computational in nature.
The Journal publishes regular articles, short communications and reviews. Reviews are normally invited by Editors or Editorial Board members. Authors of unsolicited reviews should first contact an Editor or Editorial Board member to determine whether the proposed article is within the scope of the Journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


