含有喹唑啉结构的受体相互作用蛋白激酶1 (RIPK1)抑制剂的设计、合成及其抗急性缺血性卒中(AIS)作用
IF 4.7
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
受体相互作用蛋白激酶1 (RIPK1)在坏死性坏死中起关键作用,坏死性坏死是一种导致神经元损伤和炎症的细胞死亡的调节形式。RIPK1抑制剂已成为治疗急性缺血性中风的有希望的治疗药物。本研究基于含有喹唑啉和苯并三唑基团的新型支架,设计并合成了一系列新的RIPK1抑制剂。然后在细胞水平上评估它们的有效抑制活性,RIPK1对RIPK3的选择性和抗坏死活性。值得注意的是,化合物9b成为最佳候选,通过抑制坏死坏死途径中RIPK1、RIPK3和混合谱系激酶结构域样假激酶(MLKL)的磷酸化,提供实质性的神经保护。大鼠大脑中动脉闭塞(MCAO)模型的体内评估显示,化合物9b具有神经保护作用。对该化合物的初步评估显示其毒性低,代谢谱稳定,具有良好的血脑屏障渗透性,突出了其作为AIS治疗剂的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
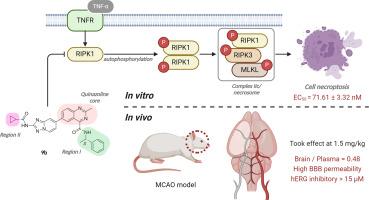
Design, synthesis, anti-acute ischemic stroke (AIS) effect of receptor-interacting protein kinase 1 (RIPK1) inhibitors containing quinazoline structure
Receptor-interacting protein kinase 1 (RIPK1) plays a pivotal role in necroptosis, a regulated form of cell death that contributes to neuronal damage and inflammation. RIPK1 inhibitors have emerged as promising therapeutic agents in the treatment of acute ischemic stroke. In this study, a series of novel RIPK1 inhibitors were designed and synthesized based on a new scaffold containing quinazoline and benzotriazole moieties. Their potent inhibitory activity, selectivity for RIPK1 over RIPK3 and anti-necroptotic activity at the cellular level were then evaluated. Notably, compound 9b emerged as an optimal candidate, offering substantial neuroprotection by inhibiting the phosphorylation of RIPK1, RIPK3, and mixed lineage kinase domain-like pseudokinase (MLKL) within the necroptosis pathway. In vivo evaluations in a rat middle cerebral artery occlusion (MCAO) model highlighted compound 9b's neuroprotective effect. Initial assessments of the compound revealed its low toxicity, stable metabolic profile, and favorable permeability across the blood-brain barrier, highlighting its promising potential as a therapeutic agent for AIS.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic Chemistry
生物-生化与分子生物学
CiteScore
9.70
自引率
3.90%
发文量
679
审稿时长
31 days
期刊介绍:
Bioorganic Chemistry publishes research that addresses biological questions at the molecular level, using organic chemistry and principles of physical organic chemistry. The scope of the journal covers a range of topics at the organic chemistry-biology interface, including: enzyme catalysis, biotransformation and enzyme inhibition; nucleic acids chemistry; medicinal chemistry; natural product chemistry, natural product synthesis and natural product biosynthesis; antimicrobial agents; lipid and peptide chemistry; biophysical chemistry; biological probes; bio-orthogonal chemistry and biomimetic chemistry.
For manuscripts dealing with synthetic bioactive compounds, the Journal requires that the molecular target of the compounds described must be known, and must be demonstrated experimentally in the manuscript. For studies involving natural products, if the molecular target is unknown, some data beyond simple cell-based toxicity studies to provide insight into the mechanism of action is required. Studies supported by molecular docking are welcome, but must be supported by experimental data. The Journal does not consider manuscripts that are purely theoretical or computational in nature.
The Journal publishes regular articles, short communications and reviews. Reviews are normally invited by Editors or Editorial Board members. Authors of unsolicited reviews should first contact an Editor or Editorial Board member to determine whether the proposed article is within the scope of the Journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


