钌(II)酚酸的对酰基配合物:合成、化学表征、硅生物分子相互作用和细胞毒性研究
IF 3.2
2区 化学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
近年来,钌配合物因具有良好的抗癌潜力而成为药物化学研究的热点。本文研究了两种钌(II)-对伞花烃基酚酸的设计、合成和化学性质。结构分析表明,Ru(II)配合物具有“钢琴凳”配位几何结构,由一个键合芳烃、两个sigma键合的三元氧原子和与Ru(II)相连的活性氯组成。对配合物的光学性质进行了研究,在270 nm和320 nm处分别出现了吸收峰,这是由于配体内部电荷跃迁和金属到配体电荷跃迁的贡献。Ru(II)配合物与小牛胸腺DNA (CT-DNA)和牛血清白蛋白(BSA)的结合性质是非共价的,Ru-1和Ru-2配合物与CT-DNA和BSA的结合常数分别为3.6 × 103 M -1、2.5 × 106 M -1和1 × 106 M -1、9.9 × 104 M -1。还观察到,在Ru(II)配合物的存在下,溴化乙啶(EtBr)通过插层从CT-DNA上竞争性地移位,这在粘度和硅研究中得到了很好的支持。这些Ru(II)复合物对多种癌细胞系(HeLa、MDA-MB-231、MCF-7、Hep G2)和一种人胚胎肾细胞(HEK-293)进行了细胞毒性研究。在体外研究了Ru(II)复合物对HeLa、MCF-7、Hep G2和MDA-MB-231细胞的细胞毒性。结果表明,与顺铂相比,Ru-1和Ru-2(范围为2.5 ~ 8.4 μM)对癌细胞具有相当的效力和选择性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
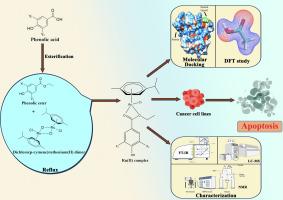
Ruthenium(II) Paracymene complexes of phenolic acids: Synthesis, chemical characterization, in-silico biomolecular interaction and cytotoxicity studies
Presently, Ruthenium (Ru) complexes drawn a significant attention in medicinal chemistry researchowing to their promising anti-cancer potential. The present research dealt with design, synthesis, and chemical characterization of two ruthenium (II)-p-cymene based phenolic acid. The structural analysis revealsthat the Ru(II) complexes acquire a ‘piano stool’ coordination geometry comprises of one bound arene, two sigma bonded esteric oxygen atoms, and labile chlorine linked to Ru(II). The optical properties of these complexes were studied,which showed absorption peaks at 270 nm and 320 nm, which are due to the contribution of intra-ligand charge transitions and metal-to-ligand charge transitions, respectively. The binding interaction of the Ru(II) complexes with calf thymus DNA (CT-DNA) and bovine serum albumin (BSA) is non-covalent in nature and the binding constants of Ru-1 and Ru-2 complexes with CT-DNA and BSA were found to be 3.6 × 103 M−1,2.5 × 106 M−1 and 1 × 106 M−1, 9.9 × 104 M−1respectively. It is also observed that in presence of Ru(II) complexes, ethidium bromide (EtBr) was competitively displaced from CT-DNA through intercalation, which is well supported by viscosity and in silico studies. The cytotoxicity study of these Ru(II) complexes was conducted with multiple cancer cell lines (HeLa, MDA-MB-231, MCF-7, Hep G2) and one human embryonic kidney cell (HEK-293). Both Ru(II) complexes were investigated in-vitro for cytotoxicity against HeLa, MCF-7, Hep G2 and MDA-MB-231 cells. The obtained results show that Ru-1 and Ru-2 (ranging from 2.5 to 8.4 μM) exhibits considerable potency and selectivity towards cancer cell lines in compare to cisplatin.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Inorganic Biochemistry
生物-生化与分子生物学
CiteScore
7.00
自引率
10.30%
发文量
336
审稿时长
41 days
期刊介绍:
The Journal of Inorganic Biochemistry is an established international forum for research in all aspects of Biological Inorganic Chemistry. Original papers of a high scientific level are published in the form of Articles (full length papers), Short Communications, Focused Reviews and Bioinorganic Methods. Topics include: the chemistry, structure and function of metalloenzymes; the interaction of inorganic ions and molecules with proteins and nucleic acids; the synthesis and properties of coordination complexes of biological interest including both structural and functional model systems; the function of metal- containing systems in the regulation of gene expression; the role of metals in medicine; the application of spectroscopic methods to determine the structure of metallobiomolecules; the preparation and characterization of metal-based biomaterials; and related systems. The emphasis of the Journal is on the structure and mechanism of action of metallobiomolecules.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


