水中羟基自由基降解羟基二苯甲酮的理论研究:微观反应机理、动力学、毒性
IF 3
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
本研究采用DFT M062X/6-311G (d, p)方法在分子水平上解释了羟基自由基(•OH)与四种二苯甲酮的微观反应机理。结果表明,最有利的反应机理是自由基加合物形成(RAF)反应。的反应速率常数K•哦,BP1(2.18×109−1−1)在凯西•哦,2 hb(4.35×108−1−1)在凯西•哦,4 dhb(1.23×108−1−1)在K•哦,4 hb(7.08×107−1−1)。基于此,我们建立了一个动力学模型来比较两种高级氧化过程(AOPs)。结果表明,UV/H2O2法对二苯甲酮类化合物的降解效率比Fenton法提高16.0 ~ 53.8%。本研究的目的是更深入地了解二苯甲酮衍生物羟基降解的基本原理,并为实际污水处理过程中的工艺选择提供理论参考。本文章由计算机程序翻译,如有差异,请以英文原文为准。
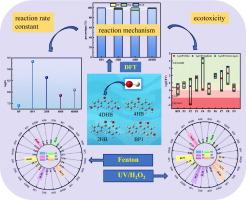
Theoretical study on the degradation of hydroxybenzophenones by hydroxyl radical in water: Microscopic reaction mechanism, kinetics, toxicity
This study used the DFT M062X/6-311G (d, p) method to explain the microscopic reaction mechanism of hydroxyl radical (•OH) with four benzophenones at the molecular level. The results showed that the most favorable reaction mechanism is the radical adduct formation (RAF) reaction. The order of reaction rate constants is K•OH, BP1 (2.18 × 109 M−1 s−1) > K•OH, 2HB (4.35 × 108 M−1 s−1) > K•OH, 4DHB (1.23 × 108 M−1 s−1) > K•OH, 4HB (7.08 × 107 M−1 s−1). Based on this, we established a kinetic model to compare two advanced oxidation processes (AOPs). The results showed that the degradation efficiency of benzophenones by the UV/H2O2 process was 16.0–53.8 % higher than that of the Fenton method. The purpose of this study is to gain a deeper understanding of the basic principles of hydroxyl degradation of benzophenone derivatives, and to provide a theoretical reference for process selection in the actual sewage treatment process.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Computational and Theoretical Chemistry
CHEMISTRY, PHYSICAL-
CiteScore
4.20
自引率
10.70%
发文量
331
审稿时长
31 days
期刊介绍:
Computational and Theoretical Chemistry publishes high quality, original reports of significance in computational and theoretical chemistry including those that deal with problems of structure, properties, energetics, weak interactions, reaction mechanisms, catalysis, and reaction rates involving atoms, molecules, clusters, surfaces, and bulk matter.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


