具有抗神经退行性变抗炎潜能的tau蛋白聚集抑制剂
IF 4.7
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
阿尔茨海默病(AD)的特点是两个病理标志:细胞外β-淀粉样斑块和细胞内由聚集的Tau蛋白形成的神经原纤维缠结(nft)。正在进行的研究重点是确定阻断Tau聚集或破坏预形成原纤维稳定的抑制剂。在这项研究中,我们评估了罗丹宁(RDN)、蒽醌(AQ)、苯胺嘧啶(PhNH2)、苯基噻唑肼(PTH)和苯并噻唑(BZT)的衍生物作为Tau聚集的潜在调节剂。我们利用钯和铜催化的偶联反应合成了目标化合物,并通过核磁共振谱证实了它们的结构。硫黄素T荧光分析和1H15N异核单量子相干(HSQC)核磁共振实验表明,PhNH2和PTH通过与关键六肽基序相互作用有效抑制Tau聚合。此外,AQ、RDN和PhNH2在lps刺激的单核细胞中降低了iNOS和COX-2的表达,强调了它们的抗炎活性。这些发现表明PhNH2和PTH是调节Tau聚集的有希望的候选者,PhNH2具有双重抗聚集和抗炎特性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
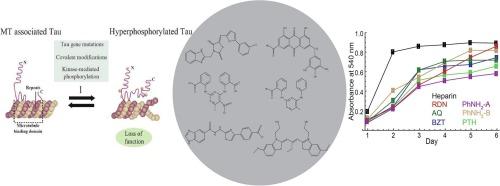
Aggregation inhibitors of tau protein with anti-inflammatory potential against neurodegeneration
Alzheimer's disease (AD) is characterized by two pathological hallmarks: extracellular β-amyloid plaques and intracellular neurofibrillary tangles (NFTs) formed by aggregated Tau protein. Ongoing research focuses on identifying inhibitors that either block Tau aggregation or destabilize pre-formed fibrils. In this study, we evaluated derivatives of Rhodanine (RDN), Anthraquinone (AQ), Phenylaminopyrimidine (PhNH2), Phenylthiazol hydrazide (PTH) and Benzothiazole (BZT) as potential modulators of Tau aggregation. We synthesized target compounds using Pd- and Cu-catalyzed coupling reactions and confirmed their structures via NMR spectroscopy. Thioflavin T fluorescence assays and 1H![]() 15N Heteronuclear Single Quantum Coherence (HSQC) NMR experiments demonstrated that PhNH2 and PTH effectively inhibited Tau polymerization by interacting with key hexapeptide motifs. Additionally, AQ, RDN, and PhNH2 reduced iNOS and COX-2 expression in LPS-stimulated monocytes, underscoring their anti-inflammatory activity. These findings suggest PhNH2 and PTH as promising candidates for modulating Tau aggregation, with PhNH2 exhibiting dual anti-aggregation and anti-inflammatory properties.
15N Heteronuclear Single Quantum Coherence (HSQC) NMR experiments demonstrated that PhNH2 and PTH effectively inhibited Tau polymerization by interacting with key hexapeptide motifs. Additionally, AQ, RDN, and PhNH2 reduced iNOS and COX-2 expression in LPS-stimulated monocytes, underscoring their anti-inflammatory activity. These findings suggest PhNH2 and PTH as promising candidates for modulating Tau aggregation, with PhNH2 exhibiting dual anti-aggregation and anti-inflammatory properties.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic Chemistry
生物-生化与分子生物学
CiteScore
9.70
自引率
3.90%
发文量
679
审稿时长
31 days
期刊介绍:
Bioorganic Chemistry publishes research that addresses biological questions at the molecular level, using organic chemistry and principles of physical organic chemistry. The scope of the journal covers a range of topics at the organic chemistry-biology interface, including: enzyme catalysis, biotransformation and enzyme inhibition; nucleic acids chemistry; medicinal chemistry; natural product chemistry, natural product synthesis and natural product biosynthesis; antimicrobial agents; lipid and peptide chemistry; biophysical chemistry; biological probes; bio-orthogonal chemistry and biomimetic chemistry.
For manuscripts dealing with synthetic bioactive compounds, the Journal requires that the molecular target of the compounds described must be known, and must be demonstrated experimentally in the manuscript. For studies involving natural products, if the molecular target is unknown, some data beyond simple cell-based toxicity studies to provide insight into the mechanism of action is required. Studies supported by molecular docking are welcome, but must be supported by experimental data. The Journal does not consider manuscripts that are purely theoretical or computational in nature.
The Journal publishes regular articles, short communications and reviews. Reviews are normally invited by Editors or Editorial Board members. Authors of unsolicited reviews should first contact an Editor or Editorial Board member to determine whether the proposed article is within the scope of the Journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


