杜氏肌营养不良患者基因治疗交付和监测的共识建议和考虑
IF 2.8
4区 医学
Q2 CLINICAL NEUROLOGY
引用次数: 0
摘要
基因转移治疗是治疗杜氏肌营养不良症(DMD)的一项重大进展。随着临床使用的扩大,迫切需要制定标准化、循证和实践指导方针,以确保安全、公平地提供这种产品和类似产品。由肌萎缩症协会和肌萎缩症家长项目协调的一组临床医生和研究人员制定了这些共识指南,概述了患者选择、机构准备、监测和不良事件管理的建议,特别是在治疗后的前三个月。该文件强调了经验丰富的多学科团队、实时安全监测和透明报告的重要性,以支持患者安全和治疗后临床医生的决策。虽然美国食品和药物管理局只批准了一种用于治疗DMD患者的基因治疗产品,但这些建议可能适用于临床开发中的其他产品。目前,在长期安全性、耐久性和最佳给药时间方面,特别是对于晚期疾病患者,仍存在重大知识空白。研究人员并不完全了解联合治疗和遗传背景如何影响对基因治疗的反应。为了解决这些差距,持续的真实世界数据收集、跨中心合作和灵活适应临床方案至关重要。虽然这些指南主要基于临床专业知识,而不是已建立的证据,但该指南为支持对DMD患者进行基因治疗提供了基础。随着该领域的发展,持续的改进对于最大限度地提高效益、降低风险和为未来的护理标准提供信息至关重要。本文章由计算机程序翻译,如有差异,请以英文原文为准。
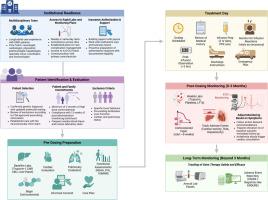
Consensus recommendations and considerations for the delivery and monitoring of gene therapy in patients with Duchenne muscular dystrophy
Gene transfer therapy represents a major advancement in the treatment of patients with Duchenne muscular dystrophy (DMD). As clinical use expands, there is an urgent need for standardized, evidence and practice-informed guidelines to ensure safe and equitable delivery of this and similar products. A group of clinicians and researchers, coordinated by the Muscular Dystrophy Association and Parent Project Muscular Dystrophy, developed these consensus guidelines to outline recommendations for patient selection, institutional readiness, monitoring, and adverse event management, particularly in the first three months after treatment. This document emphasizes the importance of experienced multidisciplinary teams, real-time safety surveillance, and transparent reporting to support patient safety and clinician decision-making after treatment.
While the Food and Drug Administration has approved only one gene therapy product for the treatment of patients with DMD, these recommendations may potentially apply to other products in clinical development. Currently, significant knowledge gaps remain regarding long-term safety, durability, and optimal timing of dosing, particularly for patients with advanced disease. Researchers do not fully understand how combination therapies and genetic background may impact the response to gene therapy. To address these gaps, ongoing real-world data collection, cross-center collaboration, and flexible adaptation of clinical protocols are essential. While these guidelines are based primarily on clinical expertise rather than well-established evidence, the guideline provides a foundation to support administration of gene therapy for patients with DMD. As the field evolves, continued refinement will be essential to maximize benefit, reduce risk, and inform future standards of care.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Neuromuscular Disorders
医学-临床神经学
CiteScore
4.60
自引率
3.60%
发文量
543
审稿时长
53 days
期刊介绍:
This international, multidisciplinary journal covers all aspects of neuromuscular disorders in childhood and adult life (including the muscular dystrophies, spinal muscular atrophies, hereditary neuropathies, congenital myopathies, myasthenias, myotonic syndromes, metabolic myopathies and inflammatory myopathies).
The Editors welcome original articles from all areas of the field:
• Clinical aspects, such as new clinical entities, case studies of interest, treatment, management and rehabilitation (including biomechanics, orthotic design and surgery).
• Basic scientific studies of relevance to the clinical syndromes, including advances in the fields of molecular biology and genetics.
• Studies of animal models relevant to the human diseases.
The journal is aimed at a wide range of clinicians, pathologists, associated paramedical professionals and clinical and basic scientists with an interest in the study of neuromuscular disorders.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


