水中外消旋四n -烷基化乙二醇的溶解/溶剂化的温度依赖焓和热容效应:溶剂H/ d同位素取代的影响
IF 5.2
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
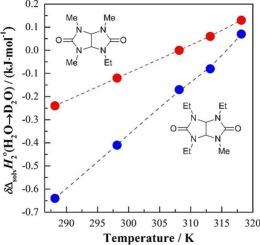
在T =(288.15, 298.15, 308.15, 313.15和318.15)K和环境压力下,用量热法测定了有药理前景的三甲基乙基乙二醇脲(TriMEGU)和三乙基甲基乙二醇脲(TriEMGU)在普通(H2O)和重(D2O)水中的标准摩尔焓和热容。估算了相应的溶剂d20 - h2o同位素效应(IEs)。TriMEGU溶解(∆solH2〇)在水介质中的焓效应在(296.15-297.15)K处经历负向正的符号反转。∆solH2〇中的IE也是如此,唯一的区别是这种反转发生在约308.15 K处。反过来,对于TriEMGU,水H/D同位素物中的∆solH2〇T值和相应的IEs均为负,直至~ 315.15 K。利用前人的量热实验结果,讨论了所研究的每一种双环溶质的水化行为,并与相关的手性二甲基二乙基糖脲(反式dmdegu,作为镇静剂)的水化行为进行了比较。研究表明,溶质水化过程中亲疏水力的温度依赖平衡不仅取决于外消旋四烷基化乙二醇衍生物分子中体积较大的烷基取代基的数量,还取决于它们的n位的立体化学特性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Temperature-dependent enthalpic and heat capacity effects of solution/solvation of racemic tetra-N-alkylated glycolurils in water: Influence of the solvent H/D-isotope substitution
The standard molar enthalpies and heat capacities of dissolution of pharmacologically promising trimethylethylglycoluril (TriMEGU) and triethylmethylglycoluril (TriEMGU) in ordinary (H2O) and heavy (D2O) water were determined calorimetrically at T = (288.15, 298.15, 308.15, 313.15, and 318.15) K and ambient pressure. The corresponding solvent D2O–H2O isotope effects (IEs) in these quantities were estimated. The enthalpic effects of TriMEGU dissolution () in the aqueous medium experience a negative-to-positive sign inversion at (296.15–297.15) K. The same goes for the IE in , with the only difference being that such an inversion occurs at ca. 308.15 K. In turn, for TriEMGU, both the values in water H/D isotopologues and the corresponding IEs were found to be negative up to ∼315.15 K. The hydration behavior of each of bicyclic solutes under study was discussed in comparison with that for the related chiral dimethyldiethylglycoluril (trans-DMDEGU, being the tranquilizer albicar) using the results of previously performed calorimetric experiments. It is established that the temperature-dependent balance of hydrophilic and hydrophobic forces in the process of a solute hydration is determined not only by the number of bulkier alkyl substituents in the molecule of the racemic tetraalkylated glycoluril-derivative, but also by the stereochemical peculiarities of their N-locations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Molecular Liquids
化学-物理:原子、分子和化学物理
CiteScore
10.30
自引率
16.70%
发文量
2597
审稿时长
78 days
期刊介绍:
The journal includes papers in the following areas:
– Simple organic liquids and mixtures
– Ionic liquids
– Surfactant solutions (including micelles and vesicles) and liquid interfaces
– Colloidal solutions and nanoparticles
– Thermotropic and lyotropic liquid crystals
– Ferrofluids
– Water, aqueous solutions and other hydrogen-bonded liquids
– Lubricants, polymer solutions and melts
– Molten metals and salts
– Phase transitions and critical phenomena in liquids and confined fluids
– Self assembly in complex liquids.– Biomolecules in solution
The emphasis is on the molecular (or microscopic) understanding of particular liquids or liquid systems, especially concerning structure, dynamics and intermolecular forces. The experimental techniques used may include:
– Conventional spectroscopy (mid-IR and far-IR, Raman, NMR, etc.)
– Non-linear optics and time resolved spectroscopy (psec, fsec, asec, ISRS, etc.)
– Light scattering (Rayleigh, Brillouin, PCS, etc.)
– Dielectric relaxation
– X-ray and neutron scattering and diffraction.
Experimental studies, computer simulations (MD or MC) and analytical theory will be considered for publication; papers just reporting experimental results that do not contribute to the understanding of the fundamentals of molecular and ionic liquids will not be accepted. Only papers of a non-routine nature and advancing the field will be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


