mir -743a-3p介导的肝脏GSTM1消除通过抑制Sp1核易位和SIRT1表达加重对乙酰氨基酚诱导的急性肝损伤
IF 8.2
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
对乙酰氨基酚(APAP)过量是药物性肝损伤(DILI)的主要原因,谷胱甘肽s -转移酶1 (GSTM1)是一种参与药物代谢和解毒的II期酶,与DILI的发生有关。然而,肝GSTM1在apap诱导的肝损伤中的作用尚不完全清楚。本研究旨在探讨肝GSTM1在apap诱导的肝毒性中的作用。采用apap诱导的小鼠肝损伤模型,通过腺相关病毒-8 (AAV8)介导的RNA递送实现肝脏特异性GSTM1和/或miR-743a-3p的沉默。我们的数据显示,在apap处理的小鼠中,GSTM1的表达显著下调。APAP诱导miR-743a-3p上调,直接抑制GSTM1表达,加重肝损伤、氧化损伤和炎症。肝脏特异性miR-743a-3p敲低可挽救GSTM1敲低并减轻肝损伤。在机制上,GSTM1与Sp1相互作用并促进其s -谷胱甘肽化。GSTM1缺失会降低Sp1 s -谷胱甘肽化,损害其核易位和转录活性,从而干扰sirtuin 1 (SIRT1)的表达。激活SIRT1可显著减轻GSTM1敲低引起的肝损伤。我们的研究结果表明,mir -743a-3p介导的GSTM1沉默通过破坏GSTM1- sp1 - sirt1轴加剧了apap诱导的肝损伤。本文章由计算机程序翻译,如有差异,请以英文原文为准。
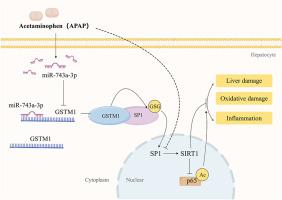
MiR-743a-3p-mediated hepatic GSTM1 elimination aggravates acetaminophen-induced acute liver injury by inhibiting Sp1 nuclear translocation and SIRT1 expression
Acetaminophen (APAP) overdose is a leading cause of drug-induced liver injury (DILI), and glutathione S-transferase mu 1 (GSTM1), a phase II enzyme involved in drug metabolism and detoxification, is associated with DILI incidence. However, the role of hepatic GSTM1 in APAP-induced liver injury is not fully understood. This study aimed to explore the role of hepatic GSTM1 in APAP-induced hepatotoxicity. A mouse model of APAP-induced liver injury was employed, and liver-specific GSTM1 and/or miR-743a-3p silencing was achieved via adeno-associated virus-8 (AAV8)-mediated RNA delivery. Our data showed that GSTM1 expression was significantly downregulated in APAP-treated mice. APAP induced miR-743a-3p upregulation, which directly suppressed GSTM1 expression, exacerbating liver injury, oxidative damage, and inflammation. Liver-specific miR-743a-3p knockdown rescued GSTM1 knockdown and mitigated liver injury. Mechanistically, GSTM1 interacted with Sp1 and promoted its S-glutathionylation. Loss of GSTM1 decreased Sp1 S-glutathionylation, impairing its nuclear translocation and transcriptional activity, which interfered with sirtuin 1 (SIRT1) expression. Activation of SIRT1 significantly alleviated GSTM1 knockdown-induced liver injury. Our findings suggest that miR-743a-3p-mediated GSTM1 silencing exacerbates APAP-induced liver injury through disruption of the GSTM1-Sp1-SIRT1 axis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


