正丁烷脱氢生成1,3-丁二烯。第3部分:焦化铬-氧化铝催化剂再生动力学
IF 1.3
Q4 ENGINEERING, CHEMICAL
引用次数: 0
摘要
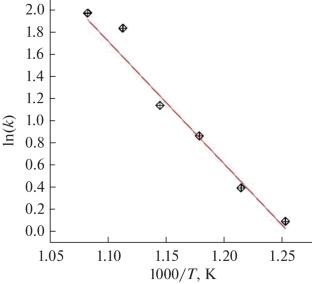
用K-CrOx /γ-Al2O3催化剂催化正丁烷直接脱氢制丁二烯1,3,研究了525 ~ 650℃下焦炭的燃烧动力学。结果表明,在研究条件下,焦炭对氧和焦炭的燃烧反应符合一级动力学方程,表观活化能为~93 kJ/mol。计算结果与试验数据吻合,证实了动力学模型的充分性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
n-Butane Dehydrogenation to 1,3-Butadiene. Part 3: Kinetics of Coked Chromia–Alumina Catalyst Regeneration
The kinetics of coke combustion at temperatures of 525–650°C on a K–CrOx/γ-Al2O3 catalyst for the direct dehydrogenation of n-butane to butadiene-1,3, which is an analogue of a commercial catalyst, has been studied. It has been shown that under the studied conditions, the coke combustion reaction is described by a first-order kinetic equation with respect oxygen and coke at an apparent activation energy of ~93 kJ/mol. The adequacy of the kinetic model has been confirmed by agreement between the calculated results and the test data.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Catalysis in Industry
ENGINEERING, CHEMICAL-
CiteScore
1.30
自引率
14.30%
发文量
21
期刊介绍:
The journal covers the following topical areas:
Analysis of specific industrial catalytic processes: Production and use of catalysts in branches of industry: chemical, petrochemical, oil-refining, pharmaceutical, organic synthesis, fuel-energetic industries, environment protection, biocatalysis; technology of industrial catalytic processes (generalization of practical experience, improvements, and modernization); technology of catalysts production, raw materials and equipment; control of catalysts quality; starting, reduction, passivation, discharge, storage of catalysts; catalytic reactors.Theoretical foundations of industrial catalysis and technologies: Research, studies, and concepts : search for and development of new catalysts and new types of supports, formation of active components, and mechanochemistry in catalysis; comprehensive studies of work-out catalysts and analysis of deactivation mechanisms; studies of the catalytic process at different scale levels (laboratory, pilot plant, industrial); kinetics of industrial and newly developed catalytic processes and development of kinetic models; nonlinear dynamics and nonlinear phenomena in catalysis: multiplicity of stationary states, stepwise changes in regimes, etc. Advances in catalysis: Catalysis and gas chemistry; catalysis and new energy technologies; biocatalysis; nanocatalysis; catalysis and new construction materials.History of the development of industrial catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


