KCl-AlCl3熔体吸附四氯化锆在四氯化锆和四氯化铪萃取精馏中的应用
IF 0.6
4区 工程技术
Q4 ENGINEERING, CHEMICAL
引用次数: 0
摘要
在324 ~ 424℃的温度范围内,采用不同类型的塔架,在中试装置上研究了KCl-AlCl3-ZrCl4熔体吸附ZrCl4蒸气的过程。在研究的温度和压力范围内,建立了ZrCl4平衡浓度与温度的关系。确定了达到平衡浓度所需的托盘数。对萃取ZrCl4和HfCl4精馏过程中回流冷凝器的操作方式提出了建议。本文章由计算机程序翻译,如有差异,请以英文原文为准。
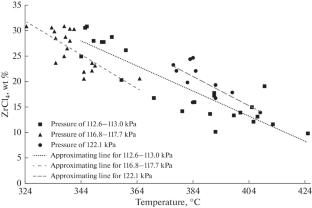
Absorption of Zirconium Tetrachloride by a KCl–AlCl3 Melt as Applied to the Extractive Distillation of Zirconium and Hafnium Tetrachlorides
The process of ZrCl4 vapor absorption by a KCl–AlCl3–ZrCl4 melt is studied on a pilot setup within a temperature range of 324–424°C in columns of different types. The dependence of the equilibrium ZrCl4 concentration on temperature is established within the studied range of temperatures and pressures. The number of trays required to attain the equilibrium concentration is determined. Some recommendations on the reflux condenser operation regimes in the process of extractive ZrCl4 and HfCl4 distillation are given.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
1.20
自引率
25.00%
发文量
70
审稿时长
24 months
期刊介绍:
Theoretical Foundations of Chemical Engineering is a comprehensive journal covering all aspects of theoretical and applied research in chemical engineering, including transport phenomena; surface phenomena; processes of mixture separation; theory and methods of chemical reactor design; combined processes and multifunctional reactors; hydromechanic, thermal, diffusion, and chemical processes and apparatus, membrane processes and reactors; biotechnology; dispersed systems; nanotechnologies; process intensification; information modeling and analysis; energy- and resource-saving processes; environmentally clean processes and technologies.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


