(R)-(-)-苯甘二醇手性哌啶-2,4-二酮分子内环化研究
IF 0.9
4区 化学
Q4 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在这项工作中,我们描述了由(R)-(-)-苯基甘二醇衍生的手性(R)-(-)-胡椒碱-2,4-二酮的合成,这是一种有价值的非对映选择性合成胡椒碱生物碱的基石。关键步骤包括分子内环化:当亲电试剂是酯时,反应通过形成格氏中间体进行,而当亲电试剂是腈时,环化遵循布莱斯型机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
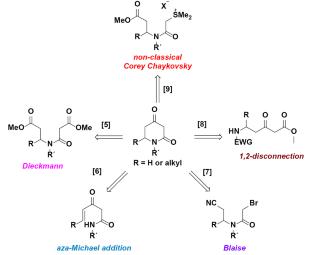
Intramolecular Cyclization of Grignard or Blaise Intermediates for the Synthesis of a Chiral Piperidine-2,4-dione Derived from (R)-(‒)-Phenylglycinol
In this work, we describe the synthesis of chiral (R)-(‒)-piperidine-2,4-dione derived from (R)-(‒)-phenylglycinol, a valuable building block for the diastereoselective synthesis of piperidine alkaloids. The key step involves intramolecular cyclization: when the electrophile is an ester, the reaction proceeds via the formation of a Grignard intermediate, whereas when the electrophile is a nitrile, the cyclization follows a Blaise-type mechanism.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
1.40
自引率
25.00%
发文量
139
审稿时长
3-6 weeks
期刊介绍:
Russian Journal of Organic Chemistry is an international peer reviewed journal that covers all aspects of modern organic chemistry including organic synthesis, theoretical organic chemistry, structure and mechanism, and the application of organometallic compounds in organic synthesis.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


