二苯甲酰甲烷在二硒和四氢吡啶-3-碳腈合成中的应用
IF 0.9
4区 化学
Q4 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在氩气条件下,以氰硒乙酰胺、呋喃-2或噻吩-2-乙醛和二苯甲酰甲烷为原料,在乙醇中加入过量的哌啶或啉,合成了3-氰基-6-羟基-1,4,5,6-四氢吡啶-2-硒酸铵,然后得到了2-烷基硒和四氢吡啶-3-碳腈。用x射线衍射分析了后两种产物的结构。本文章由计算机程序翻译,如有差异,请以英文原文为准。
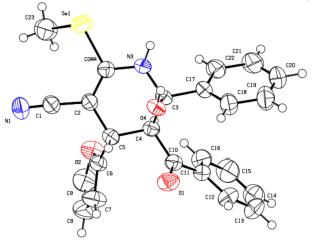
Dibenzoylmethane in the Synthesis of Selenium Di- and Tetrahydropyridine-3-carbonitriles
3-Cyano-6-hydroxy-1,4,5,6-tetrahydropyridine-2-ammonium selenolates have been synthesized by the reaction of cyanoselenoacetamide, furan-2- or thiophene-2-carbaldehyde, and dibenzoylmethane in ethanol in the presence of an excess of piperidine or morpholine under argon and then used to obtain 2-alkylselenodi- and tetrahydropyridine-3-carbonitriles. The structures of the latter products were analyzed by X-ray diffraction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
1.40
自引率
25.00%
发文量
139
审稿时长
3-6 weeks
期刊介绍:
Russian Journal of Organic Chemistry is an international peer reviewed journal that covers all aspects of modern organic chemistry including organic synthesis, theoretical organic chemistry, structure and mechanism, and the application of organometallic compounds in organic synthesis.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


