与滴铸金纳米颗粒相比,在丝网印刷碳电极上电沉积的金纳米颗粒表现出不同的电化学和催化性能
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
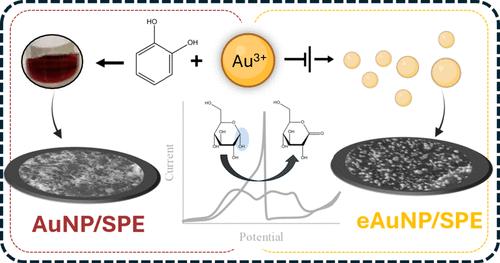
利用金纳米颗粒(AuNPs)制造电极通常依赖于化学合成的AuNP滴铸或电化学还原Au3+直接到碳电极支架上。这种aunp功能化电极已经广泛和普遍地用于各种应用,包括传感和催化。然而,目前还没有系统的研究集中在综合评价两种类型的电极表面(滴铸型和电化学沉积型)的电化学性能和电催化活性方面。在这项研究中,我们对丝网印刷碳电极(SPE)进行了修饰,制造了两个不同的电极表面,AuNP/SPE和eAuNP/SPE,以广泛比较它们的电化学和催化性能。具体来说,通过循环伏安法(CV)对两种电极在酸性和碱性电解质中进行了表征,并表现出相似的电活性表面积(ECSA)、表面粗糙度和AuO层覆盖率,以及不同粒径的球形形貌。CV和电化学阻抗谱(EIS)揭示了表面的复杂性。AuNP/SPE表面是不均匀的,偏离了预期的电位与扫描速率平方根的线性关系,并且表现出与溶液氧化还原探针(铁/亚铁氰化物)相关的更低电流、更慢的扩散和更小的观察到的电子转移速率。值得注意的是,AuNP/SPE和eAuNP/SPE电极电化学性能的显著差异导致它们在葡萄糖为底物的模拟氧化酶电催化活性上存在明显差异。用AuNP/SPE氧化葡萄糖需要较高的阳极起始电位,这也导致较低的电流和较低的活性。总之,我们确定了特定的电化学性质,如表面非均质性和电子转移,作为(e) aunp功能化碳电极电催化活性的主要决定因素。在经常互换使用的电极上观察到的独特表面性质解释了不同的催化活性,这些活性可以转移到其他表面和底物上。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Electrodeposited Gold Nanoparticles on a Screen-Printed Carbon Electrode Exhibit Distinct Electrochemical and Catalytic Properties Compared to Drop-Cast Gold Nanoparticles
The utilization of Au nanoparticles (AuNPs) for electrode fabrication often relies on the drop-casting of the chemically synthesized AuNP or electrochemically reducing Au3+ directly onto a carbon electrode support. Such AuNP-functionalized electrodes have been extensively and ubiquitously used for various applications, including sensing and catalysis. However, there is no systematic study focusing on comprehensively evaluating the two types of electrode surfaces (drop-cast AuNPs vs electrochemically deposited eAuNPs) in terms of their electrochemical properties and electrocatalytic activity. In this study, we modified the screen-printed carbon electrodes (SPEs) to fabricate two distinct electrode surfaces, AuNP/SPE and eAuNP/SPE, for the extensive comparison of their electrochemical and catalytic properties. Specifically, both electrodes were characterized in acidic and basic electrolytes by cyclic voltammetry (CV) and exhibited similar electroactive surface area (ECSA), surface roughness, and AuO layer coverage, as well as spherical morphologies with different particle sizes. CV and electrochemical impedance spectroscopy (EIS) uncovered the complex nature of the surfaces. The AuNP/SPE surface was heterogeneous, deviated from the linearity expected for potential vs square-root of scan rates, and exhibited lower current, slower diffusion, and smaller observed rates of electron transfer related to the solution redox probe, ferri/ferrocyanide. Notably, the significant differences in electrochemical properties of AuNP/SPE and eAuNP/SPE electrodes resulted in stark variances in their electrocatalytic activities as oxidase-mimics with glucose as a substrate. Higher anodic onset potential was required for glucose oxidation with AuNP/SPE, which also resulted in lower current and lower activity. Altogether, we identified specific electrochemical properties, such as surface heterogeneity and electron transfer, as the dominant determinants of the electrocatalytic activities of (e)AuNP-functionalized carbon electrodes. The unique surface properties observed for often interchangeably used electrodes explain the differential catalytic activities, which may be transferrable to other surfaces and substrates.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


