外泌体miR-27a-5p通过靶向USF2/FUT8轴抑制吲哚酚硫酸盐诱导的心功能障碍。
IF 8.2
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
尿毒症心肌病(UCM)是导致血液透析患者死亡的主要原因。硫酸吲哚酚(Indoxyl sulfate, IS)是一种关键的尿毒症毒素,可激活多种信号通路,导致心脏肥大和细胞凋亡。我们之前证明了核心聚焦化(CF),一种翻译后修饰,在激活这些途径中是至关重要的。此外,外泌体介导的心脏微血管内皮细胞(CMEC)-心肌细胞(CM)串扰对UCM的进展也很重要。然而,CF修饰在is诱导的cmec衍生外泌体(IS-Exos)中引起CM损伤的特征和作用仍未被探索。我们的研究发现,血液透析患者血清IS和α1,6- focusyltransferase (FUT8)明显升高,且与心脏损伤严重程度呈正相关。利用is诱导心脏损伤的微流控芯片模型,我们观察到外泌体从cmec向CMs转移,导致CMs中线粒体损伤、肥大和凋亡,其中CF水平升高起关键作用。为了进一步研究这一点,我们在IS-Exos处理的is -小鼠中进行了FUT8敲除,并将FUT8 siRNA转染到暴露于IS-Exos的CMs中。我们发现抑制FUT8导致ARG2表达减少,从而减少活性氧(ROS),改善心脏肥大和细胞凋亡。在机制上,miR-27a-5p在IS-Exos中明显下调。IS-Exos上的CD44与CMs中的EGFR相互作用,增强心脏损伤。在体内和体外补充miR-27a-5p特异性靶向USF2,导致FUT8表达下调。这一级联反应导致ARG2的表达减少,ROS的减轻,以及心脏肥大和细胞凋亡的逆转。我们的研究结果提供了新的见解,表明靶向CF修饰可能是未来缓解UCM的一种有希望的治疗策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
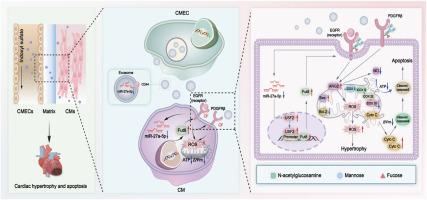
Exosomal miR-27a-5p inhibits indoxyl sulfate-induced cardiac dysfunction by targeting the USF2/FUT8 axis
Uremic cardiomyopathy (UCM) is the leading cause of hemodialysis patient mortality. Indoxyl sulfate (IS), a key uremic toxin, activates multiple signaling pathways, causing cardiac hypertrophy and apoptosis. We previously demonstrated that core fucosylation (CF), a post-translational modification, is crucial in activating these pathways. Additionally exosome-mediated cardiac microvascular endothelial cell (CMEC)-cardiomyocyte (CM) crosstalk is important for UCM progression. However, the characteristics and roles of CF modification in IS-induced CMEC-derived exosomes (IS-Exos) that cause CM injury remain unexplored. Our studies have revealed that hemodialysis patients had significantly higher serum IS and α1,6-fucosyltransferase (FUT8), which were positively correlated with the severity of cardiac injury. Using a microfluidic chip model of IS-induced cardiac injury, we visualized exosome transfer from CMECs to CMs, which caused mitochondrial impairment, hypertrophy and apoptosis in CMs, with elevated CF levels playing critical roles. To investigate this further, we performed FUT8 knockout in IS-mice treated with IS-Exos and transfected FUT8 siRNA into CMs exposed to IS-Exos. We found that the inhibition of FUT8 leads to a reduction in ARG2 expression, which consequently diminishes reactive oxygen species (ROS) and ameliorates cardiac hypertrophy and apoptosis. Mechanistically, miR-27a-5p was markedly downregulated in IS-Exos. CD44 on IS-Exos interacts with EGFR in CMs, enhancing cardiac injury. Supplementation with miR-27a-5p in vivo and in vitro specifically targets USF2, leading to a downregulation of FUT8 expression. This cascade leads to a diminished expression of ARG2, alleviation of ROS, and the reversal of cardiac hypertrophy and apoptosis. Our findings offer new insights, suggesting that targeting CF modification may represent a promising therapeutic strategy for alleviating UCM in the future.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


