口腔微生物-氧化还原-炎症轴在神经退行性变:机制的见解和治疗的观点。
IF 5.6
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
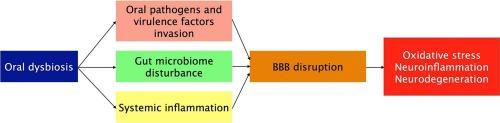
口腔微生物群是一个高度多样化和代谢活跃的生态系统,在维持口腔和全身稳态中起着关键作用。这种平衡的破坏被称为口腔生态失调,已越来越多地与神经退行性疾病(NDs)的发病机制有关,如阿尔茨海默病(AD)、帕金森病(PD)和肌萎缩侧索硬化症(ALS)。尽管精确的分子机制仍未完全确定,但越来越多的证据表明,氧化应激和氧化还原信号作为中枢介质,将微生物失衡与神经炎症反应和进行性神经元功能障碍联系起来。在这篇综述中,我们批判性地综合了口腔微生物组-脑轴的跨学科研究成果,强调氧化还原敏感途径介导口腔病原体与中枢神经系统之间的交流。我们讨论了由微生物代谢物和病原体相关分子模式产生的活性氧(ROS)如何激活各种信号级联,从而加剧神经炎症和胶质细胞激活。我们进一步评估了口腔生态失调导致血脑屏障(BBB)破坏、外周免疫启动和慢性神经免疫失调的证据。通过综合机制、细胞和临床观点,我们确定氧化应激和氧化还原信号是口腔生态失调和神经退行性变之间的关键生物学桥梁。该框架不仅强调了靶向氧化还原途径和口腔微生物组的预防和治疗策略的转化潜力,而且还强调了未来研究澄清因果关系和验证临床应用的必要性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
The oral microbiome–redox–inflammation axis in neurodegeneration: mechanistic insights and therapeutic perspectives
The oral microbiome is a highly diverse and metabolically active ecosystem that plays a pivotal role in maintaining oral and systemic homeostasis. Disruption of this balance, referred to as oral dysbiosis, has been increasingly implicated in the pathogenesis of neurodegenerative diseases (NDs) such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and amyotrophic lateral sclerosis (ALS). Although the precise molecular mechanisms remain incompletely defined, accumulating evidence indicates that oxidative stress and redox signaling act as central mediators linking microbial imbalance to neuroinflammatory responses and progressive neuronal dysfunction. In this review, we critically synthesize interdisciplinary findings on the oral microbiome–brain axis, emphasizing redox-sensitive pathways that mediate communication between oral pathogens and the central nervous system. We discuss how reactive oxygen species (ROS), generated by microbial metabolites and pathogen-associated molecular patterns, activate various signaling cascades, thereby exacerbating neuroinflammation and glial activation. We further evaluate evidence that oral dysbiosis contributes to blood–brain barrier (BBB) disruption, peripheral immune priming, and chronic neuroimmune dysregulation. By integrating mechanistic, cellular, and clinical perspectives, we identify oxidative stress and redox signaling as critical biological bridges between oral dysbiosis and neurodegeneration. This framework highlights not only the translational potential of targeting redox pathways and the oral microbiome for preventive and therapeutic strategies but also the need for future research to clarify causal relationships and validate clinical applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


