感觉神经元通过谷氨酸能神经元癌伪突触驱动胰腺癌进展
IF 44.5
1区 医学
Q1 CELL BIOLOGY
引用次数: 0
摘要
癌症在神经元输入下茁壮成长。在此,我们证明了感觉神经末梢与脑外癌(即胰腺导管腺癌(PDAC))中癌细胞之间存在伪突触连接。这些突触位点在癌细胞上选择性富集谷氨酸能n-甲基-d -天冬氨酸受体(NMDA)亚基NMDAR2D (GRIN2D),使PDAC细胞对神经元源性谷氨酸产生反应,促进肿瘤生长和扩散。有趣的是,神经元通过神经元癌伪突触中的grin2d型谷氨酸受体将共培养的PDAC细胞亚群转化为钙反应细胞。我们发现这个亚基的表达是由于在神经营养前馈回路中感觉神经支配提供的谷氨酸可用性增加。此外,干扰这些神经元癌伪突触的谷氨酸- grin2d信号显著提高了体内存活。外周癌症神经元伪突触的发现可能为癌症神经科学指导的肿瘤治疗提供机会。本文章由计算机程序翻译,如有差异,请以英文原文为准。
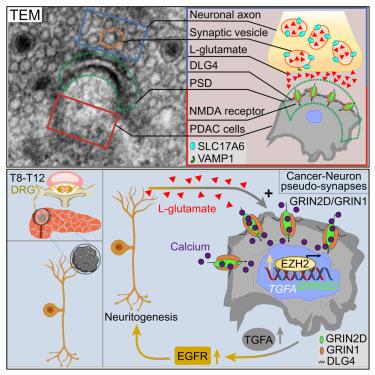
Sensory neurons drive pancreatic cancer progression through glutamatergic neuron-cancer pseudo-synapses
Cancers thrive on neuronal input. Here, we demonstrate the presence of pseudo-synaptic connections between sensory nerve endings and cancer cells in an extracerebral cancer, i.e., pancreatic ductal adenocarcinoma (PDAC). These synaptic sites exhibit a selective enrichment of the glutamatergic N-methyl-D-aspartate receptor (NMDA) receptor subunit NMDAR2D (GRIN2D) on the cancer cells, which turns PDAC cells responsive to neuron-derived glutamate and promotes tumor growth and spread. Intriguingly, neurons transform a subset of co-cultured PDAC cells into calcium-responsive cells via GRIN2D-type glutamate receptors at the neuron-cancer pseudo-synapses. We found that the expression of this subunit is due to the increased glutamate availability provided by sensory innervation in a neurotrophic feedforward loop. Moreover, interference with the glutamate-GRIN2D signaling at these neuron-cancer pseudo-synapses markedly improved survival in vivo. This discovery of peripheral cancer-neuron pseudo-synapses may provide an opportunity for cancer-neuroscience-instructed oncological therapies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cancer Cell
医学-肿瘤学
CiteScore
55.20
自引率
1.20%
发文量
179
审稿时长
4-8 weeks
期刊介绍:
Cancer Cell is a journal that focuses on promoting major advances in cancer research and oncology. The primary criteria for considering manuscripts are as follows:
Major advances: Manuscripts should provide significant advancements in answering important questions related to naturally occurring cancers.
Translational research: The journal welcomes translational research, which involves the application of basic scientific findings to human health and clinical practice.
Clinical investigations: Cancer Cell is interested in publishing clinical investigations that contribute to establishing new paradigms in the treatment, diagnosis, or prevention of cancers.
Insights into cancer biology: The journal values clinical investigations that provide important insights into cancer biology beyond what has been revealed by preclinical studies.
Mechanism-based proof-of-principle studies: Cancer Cell encourages the publication of mechanism-based proof-of-principle clinical studies, which demonstrate the feasibility of a specific therapeutic approach or diagnostic test.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


