生酮饮食通过促进肠道微生物衍生丁酸盐的产生来抑制胶质瘤的进展
IF 44.5
1区 医学
Q1 CELL BIOLOGY
引用次数: 0
摘要
生酮饮食(KD)是一种潜在的治疗胶质瘤的策略;然而,潜在的机制仍不清楚。在此,我们首先确定胶质瘤患者表现出独特的肠道微生物特征,其特征是丁酸盐产生细菌丰度降低,特别是粪肠球菌,同时丁酸盐水平降低。值得注意的是,KD重塑了肠道微生物群,特别是以粘蛋白2依赖的方式富集嗜粘杆菌,提高了丁酸盐的产生,并激活了小胶质细胞中的caspase-3。这些变化促进抗肿瘤小胶质细胞表型,最终抑制小鼠胶质瘤的进展。关键是,KD的抗胶质瘤作用被抗生素治疗明显消除;无菌条件;或特定的粘蛋白-2,小胶质细胞,或小胶质细胞caspase-3的消耗。此外,丁酸盐、嗜粘单胞菌、粪单胞菌或嗜粘单胞菌加粪单胞菌可恢复抗生素处理或无菌小鼠中kd诱导的小胶质caspase-3激活和小胶质细胞的抗肿瘤表型。这些发现强调,通过KD靶向肠道微生物群或补充丁酸盐可能是治疗胶质瘤的有效策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
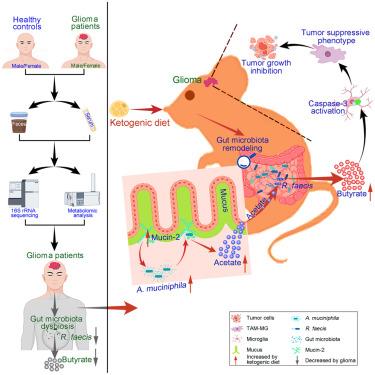
Ketogenic diet inhibits glioma progression by promoting gut microbiota-derived butyrate production
The ketogenic diet (KD) is a potential therapeutic strategy for glioma; however, the underlying mechanisms remain unclear. Herein, we first identify that glioma patients exhibit a distinct gut microbial profile characterized by reduced butyrate-producing bacteria abundance, particularly R. faecis, along with decreased butyrate levels. Notably, KD reshapes the gut microbiota especially enriching A. muciniphila in a mucin-2-dependent manner, elevates butyrate production, and activates caspase-3 in microglia. These changes promote an anti-tumor microglial phenotype, ultimately suppressing glioma progression in mice. Crucially, KD’s anti-glioma effect is notably abolished by antibiotics treatment; germ-free condition; or specific depletion of mucin-2, microglia, or microglial caspase-3. Furthermore, butyrate, A. muciniphila, R. faecis, or A. muciniphila plus R. faecis restores KD-induced microglial caspase-3 activation and the anti-tumor phenotype of microglia in antibiotics-treated or germ-free mice. These findings highlight that targeting the gut microbiota by KD or supplementing with butyrate could be an effective strategy for glioma therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cancer Cell
医学-肿瘤学
CiteScore
55.20
自引率
1.20%
发文量
179
审稿时长
4-8 weeks
期刊介绍:
Cancer Cell is a journal that focuses on promoting major advances in cancer research and oncology. The primary criteria for considering manuscripts are as follows:
Major advances: Manuscripts should provide significant advancements in answering important questions related to naturally occurring cancers.
Translational research: The journal welcomes translational research, which involves the application of basic scientific findings to human health and clinical practice.
Clinical investigations: Cancer Cell is interested in publishing clinical investigations that contribute to establishing new paradigms in the treatment, diagnosis, or prevention of cancers.
Insights into cancer biology: The journal values clinical investigations that provide important insights into cancer biology beyond what has been revealed by preclinical studies.
Mechanism-based proof-of-principle studies: Cancer Cell encourages the publication of mechanism-based proof-of-principle clinical studies, which demonstrate the feasibility of a specific therapeutic approach or diagnostic test.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


