醛作为CO释放分子:原位和非原位反应和钯催化的氨基羰基化
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
我们报告采用了一种双室反应器,利用脂肪族醛在CO释放分子(CORMs)中的作用。在光催化条件下,首先形成酰基自由基(RCO•),容易失去CO。产生的烷基自由基被用于原位共轭加成到Michael受体上(在第一个腔室),释放的CO被用于非原位钯催化的氨基羰基化(在第二个腔室),以确保100%的原子经济反应。本文章由计算机程序翻译,如有差异,请以英文原文为准。
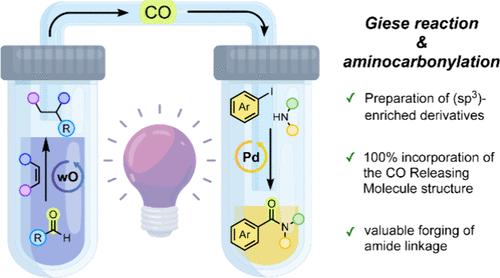
Aldehydes as CO Releasing Molecules: In Situ and Ex Situ Giese Reactions and Palladium-Catalyzed Aminocarbonylations
We report the adoption of a two-chamber reactor making use of aliphatic aldehydes in the role of CO releasing molecules (CORMs). Upon photocatalytic conditions, an acyl radical (RCO•) was first formed, prone to lose CO. The resulting alkyl radical was employed in the in situ conjugate addition onto a Michael acceptor (in the first chamber), and the released CO was employed in ex situ palladium-catalyzed aminocarbonylations (in the second chamber) to ensure a 100% atom-economical reaction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


