碳配体对重氮硼烷性质和反应性的影响
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
虽然重氮化合物和有机叠氮化物在过去的两个世纪里得到了广泛的研究,但它们的硼类似物重氮硼烷直到去年才被成功分离出来,它们的性质在很大程度上仍未被探索。本研究对重氮硼烷的合成、性质和反应性进行了系统的研究。在这项工作中,通过改变与硼配位的碳配体,将重氮硼烷库扩展到8个物种,从而确定配体电子性质与重氮硼烷行为之间的关键相关性。结果表明,增加碳烯π-酸度会减弱B-N键而增强N-N键,热稳定性显著降低。动力学研究表明,较少的π-酸性碳烯促进二级衰变机制,导致二硼氮的生成,而较多的π-酸性碳烯有利于一级二氮解离,促进硼炔的生成。电化学研究表明,氧化还原电位也与碳烯π-酸度有关。提出了三种代表性的反应性研究,其中相同的底物与不同的重氮硼烷反应产生不同的产物或通过不同的反应机制产生相同的产物。这些见解为调整重氮硼烷性质提供了一个可推广的框架,促进了它们在环加成化学和小分子活化中的应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
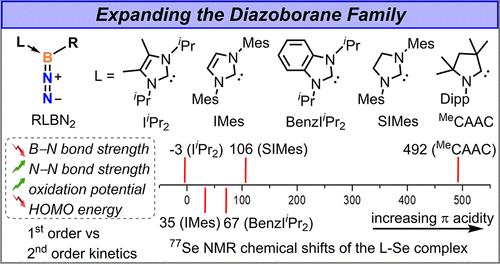
Impact of Carbene Ligands on the Properties and Reactivity of Diazoboranes
While diazo compounds and organic azides have been extensively studied over the past two centuries, their boron analogs, diazoboranes, were only successfully isolated last year, and their properties remain largely unexplored. This study presents a systematic investigation of the synthesis, properties, and reactivity of diazoboranes. In this work, the diazoborane library is expanded to eight species by varying the carbene ligands coordinated to boron, allowing the identification of key correlations between ligand electronic properties and diazoborane behavior. The results demonstrate that increasing carbene π-acidity weakens the B–N bond while strengthening the N–N bond, significantly decreasing thermal stability. Kinetic studies reveal that less π-acidic carbenes promote a second-order decay mechanism, leading to dibora-azine formation, whereas more π-acidic carbenes favor first-order dinitrogen dissociation, facilitating borylene generation. Electrochemical studies indicate that redox potentials also correlate with carbene π-acidity. Three representative reactivity studies are presented in which the same substrate reacts with different diazoboranes to yield either different products or the same product via different reaction mechanisms. These insights provide a generalizable framework for tuning diazoborane properties, facilitating their use in cycloaddition chemistry and small-molecule activation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


