肠道菌群与心血管疾病:探索微生物生态失调和代谢物在发病机制和治疗中的作用。
IF 5.1
2区 医学
Q1 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
摘要
肠道菌群是一个动态的人体微生物生态系统,在调节宿主生理和免疫功能方面起着关键作用,对心血管系统有重要影响。心血管疾病(cvd)是世界范围内导致死亡的主要原因。肠道微生物群失调与多种心血管疾病有关,如高血压、动脉粥样硬化、冠状动脉疾病、心力衰竭和心肌梗死,其机制由微生物衍生代谢物介导。关键化合物包括三甲胺n -氧化物(TMAO)(与斑块不稳定有关)、短链脂肪酸(SCFAs)(调节血压和内皮功能)、苯乙酰谷氨酰胺(PAGln)(血栓形成途径的促进剂)和胆胆酸(影响脂质代谢)。这些代谢物既是心血管疾病风险的生物标志物,也是治疗靶点。调节肠道微生物群的新策略,如精准益生菌、饮食干预(如富含纤维或多酚的饮食)和微生物酶的药理学抑制剂(如TMA裂解酶阻断剂),突出了微生物组导向治疗的潜力。然而,在阐明微生物致病途径、标准化不同人群的干预措施以及将临床前发现转化为临床实践方面仍然存在挑战。在这篇机制和翻译综述中,我们综合了目前关于肠道微生物生态失调与心血管疾病之间双向关系的证据。我们探讨了可改变的因素,包括饮食、药物治疗和生命早期微生物定植,如何重塑肠道群落,驱动全身性炎症、代谢功能障碍和血管病理。未来的研究必须优先考虑纵向人体研究、多组学整合和随机试验,以充分利用肠道微生物群在心血管疾病预防和个性化治疗中的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
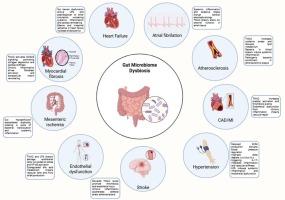
The gut microbiota and cardiovascular disease: Exploring the role of microbial dysbiosis and metabolites in pathogenesis and therapeutics
The gut microbiota, a dynamic ecosystem of microorganisms inhabiting the human body, plays a pivotal role in modulating host physiology and immune function, with intense implications for the cardiovascular system. Cardiovascular diseases (CVDs) stand out as the leading cause of mortality worldwide. Gut microbiome dysbiosis is implicated in diverse CVDs, such as hypertension, atherosclerosis, coronary artery disease, heart failure, and myocardial infarction, through mechanisms mediated by microbial-derived metabolites. Key compounds include trimethylamine N-oxide (TMAO) (linked to plaque instability), short-chain fatty acids (SCFAs) (which regulate blood pressure and endothelial function), phenylacetylglutamine (PAGln) (a promoter of thrombotic pathways), and bile acids (influencing lipid metabolism). These metabolites serve as both biomarkers of CVD risk and therapeutic targets. Emerging strategies to modulate the gut microbiota, such as precision probiotics, dietary interventions (e.g., fiber-rich or polyphenol-heavy diets), and pharmacologic inhibitors of microbial enzymes (e.g., TMA lyase blockers), highlight the potential for microbiome-directed therapies. However, challenges remain in elucidating causal microbial pathways, standardizing interventions across diverse populations, and translating preclinical findings into clinical practice. In this mechanistic and translational review, we synthesize current evidence on the bidirectional relationship between gut microbial dysbiosis and CVDs. We explore how modifiable factors, including diet, pharmacotherapy, and early-life microbial colonization, reshape gut communities, driving systemic inflammation, metabolic dysfunction, and vascular pathology. Future research must prioritize longitudinal human studies, multi-omics integration, and randomized trials to harness the gut microbiota's full potential in CVD prevention and personalized treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Life sciences
医学-药学
CiteScore
12.20
自引率
1.60%
发文量
841
审稿时长
6 months
期刊介绍:
Life Sciences is an international journal publishing articles that emphasize the molecular, cellular, and functional basis of therapy. The journal emphasizes the understanding of mechanism that is relevant to all aspects of human disease and translation to patients. All articles are rigorously reviewed.
The Journal favors publication of full-length papers where modern scientific technologies are used to explain molecular, cellular and physiological mechanisms. Articles that merely report observations are rarely accepted. Recommendations from the Declaration of Helsinki or NIH guidelines for care and use of laboratory animals must be adhered to. Articles should be written at a level accessible to readers who are non-specialists in the topic of the article themselves, but who are interested in the research. The Journal welcomes reviews on topics of wide interest to investigators in the life sciences. We particularly encourage submission of brief, focused reviews containing high-quality artwork and require the use of mechanistic summary diagrams.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


