慢性心理应激介导神经胶质- ilc3回路加重肠道炎症和抑郁。
IF 7.6
2区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
慢性心理应激可加重炎症性肠病(IBD)患者和实验性结肠炎动物模型的肠道炎症,但其潜在机制尚不清楚。在这里,我们发现了一个慢性应激介导的肠胶质- 3组先天淋巴样细胞(ILC3)回路,该回路损害先天白细胞介素(IL)-22介导的免疫保护。我们发现,应激通过激活下丘脑-垂体-肾上腺轴促进肾上腺皮质释放过量的糖皮质激素(GC)。随后,长期升高的GC水平通过与肠胶质细胞(EGCs)上的糖皮质激素受体结合,使EGCs的表型和功能失调,从而削弱胶质源性神经营养因子的产生。这些导致先天IL-22的缺乏和肠道防御的损害。同时,我们证明肠道炎症的加剧进一步释放神经炎症和焦虑/抑郁样行为的进展。我们的工作揭示了慢性心理应激加剧肠道炎症的新机制,扩大了对ILC3生物学和ILC3介导的粘膜免疫的理解。我们提出了一种基于先天il -22的治疗策略,用于治疗伴有心理合并症的IBD患者,并建议在双向脑肠沟通的背景下,压力管理是IBD护理的一个有价值的组成部分。本文章由计算机程序翻译,如有差异,请以英文原文为准。
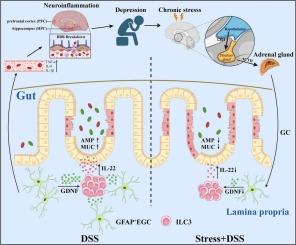
Chronic psychological stress-orchestrated glial-ILC3 circuit exacerbates intestinal inflammation and depression
Chronic psychological stress can exacerbate intestinal inflammation both in patients with inflammatory bowel disease (IBD) and animal models of experimental colitis, but the underlying mechanism remains poorly understood. Here, we identified a chronic stress-orchestrated intestinal glia-Group 3 innate lymphoid cell (ILC3) circuit that impairs innate interleukin (IL)–22-mediated immune protection. We found that stress promoted the release of excessive amounts of glucocorticoid (GC) by the adrenal cortex through activation of the hypothalamic–pituitary–adrenal axis. Subsequently, chronically elevated level of GC dysregulated the phenotype and function of enteric glial cells (EGCs) through binding to the glucocorticoid receptor on EGCs, thereby weakening glial-derived neurotrophic factor production. These lead to the deficiency of innate IL-22 and impairment of gut defense. Simultaneously, we demonstrated that exacerbation of intestinal inflammation further unleashes the progression of neuroinflammation and anxiety/depression-like behaviors. Our work sheds light on a novel mechanism by which chronic psychological stress exacerbates intestinal inflammation, expanding the understanding of ILC3 biology and ILC3-mediated mucosal immunity. We proposed an innate IL-22-based therapeutic strategy for IBD patients with psychological comorbidities, and suggested stress management as a valuable component of IBD care in the context of bidirectional brain-gut communication.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
29.60
自引率
2.00%
发文量
290
审稿时长
28 days
期刊介绍:
Established in 1987, Brain, Behavior, and Immunity proudly serves as the official journal of the Psychoneuroimmunology Research Society (PNIRS). This pioneering journal is dedicated to publishing peer-reviewed basic, experimental, and clinical studies that explore the intricate interactions among behavioral, neural, endocrine, and immune systems in both humans and animals.
As an international and interdisciplinary platform, Brain, Behavior, and Immunity focuses on original research spanning neuroscience, immunology, integrative physiology, behavioral biology, psychiatry, psychology, and clinical medicine. The journal is inclusive of research conducted at various levels, including molecular, cellular, social, and whole organism perspectives. With a commitment to efficiency, the journal facilitates online submission and review, ensuring timely publication of experimental results. Manuscripts typically undergo peer review and are returned to authors within 30 days of submission. It's worth noting that Brain, Behavior, and Immunity, published eight times a year, does not impose submission fees or page charges, fostering an open and accessible platform for scientific discourse.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


