金嘌呤通过调节胰腺导管腺癌中txnrd1相关的铁下垂促进吉西他滨敏感性。
IF 10.1
1区 医学
Q1 ONCOLOGY
引用次数: 0
摘要
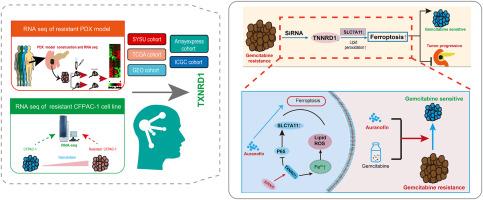
吉西他滨联合化疗仍然是胰腺导管腺癌(PDAC)的一线治疗方法。然而,许多PDAC患者对吉西他滨产生耐药性,这突出了确定致敏剂或耐药靶点的必要性。在这项研究中,我们利用患者切除的PDAC组织构建了患者来源的异种移植物(PDX)模型,并建立了稳定的吉西他滨耐药和敏感的PDX模型。对耐吉西他滨PDX模型和细胞系的RNA测序分析显示,铁离子代谢的改变显著影响了PDAC耐吉西他滨。吉西他滨耐药细胞系表现出铁离子水平的改变,这有助于减少脂质过氧化和铁下垂。为了识别吉西他滨耐药的生物标志物,我们建立了基于pdx的机器学习特征,发现它们可以区分不同患者化疗和免疫治疗的有效性。TXNRD1是一种潜在的致癌基因,可促进细胞迁移和增殖,同时抑制细胞凋亡。在机制上,TXNRD1敲低限制了P65的表达和磷酸化,导致SLC7A11缺失和铁下沉增强。这种由SLC7A11抑制诱导的激活的铁下垂,进一步抑制了PDAC的吉西他滨耐药性。TXNRD1抑制剂Auranofin可诱导铁下垂,并与吉西他滨在PDAC治疗中发挥协同作用。在耐药PDAC - PDX模型中,金嘌呤还增强了吉西他滨的抗癌作用。总的来说,TXNRD1是克服吉西他滨耐药的潜在靶点。金嘌呤可以抑制TXNRD1的激活,从而使PDAC细胞对吉西他滨敏感。欧诺芬和吉西他滨联合治疗可能对PDAC化疗具有转化潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Auranofin facilitates gemcitabine sensitivity by regulating TXNRD1-related ferroptosis in pancreatic ductal adenocarcinoma
Gemcitabine-based combination chemotherapy remains the first-line treatment for pancreatic ductal adenocarcinoma (PDAC). However, numerous patients with PDAC develop resistance to gemcitabine, highlighting the need to identify sensitizers or resistance targets. In this study, we constructed a patient-derived xenograft (PDX) model using resected PDAC tissue from patients and established stable gemcitabine-resistant and -sensitive PDX models. RNA sequencing analysis of the gemcitabine-resistant PDX model and cell lines revealed that altered iron ion metabolism significantly affected gemcitabine resistance in PDAC. Gemcitabine-resistant cell lines exhibited altered iron ion levels, which contributed to decreasing lipid peroxidation and ferroptosis. To identify biomarkers of gemcitabine resistance, we established PDX-based machine learning features and found that they may differentiate the effectiveness of chemotherapy and immunotherapy in different patients. TXNRD1 was identified as a potential oncogene that promotes cell migration and proliferation, while inhibiting cell apoptosis. Mechanistically, TXNRD1 knockdown restricted P65 expression and phosphorylation, leading to SLC7A11 depletion and enhanced ferroptosis. This activated ferroptosis, induced by SLC7A11 inhibition, further suppressed gemcitabine resistance in PDAC. Auranofin, a TXNRD1 inhibitor, induced ferroptosis and exerted synergistic effects with gemcitabine in PDAC therapy. Auranofin additionally enhanced the anticancer effects of gemcitabine in a drug-resistant PDAC PDX model. Collectively, TXNRD1 is a potential target for overcoming gemcitabine resistance. Auranofin can inhibit TXNRD1 activation, thereby sensitizing PDAC cells to gemcitabine. Combination therapy with auranofin and gemcitabine may have translational potential for PDAC chemotherapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cancer letters
医学-肿瘤学
CiteScore
17.70
自引率
2.10%
发文量
427
审稿时长
15 days
期刊介绍:
Cancer Letters is a reputable international journal that serves as a platform for significant and original contributions in cancer research. The journal welcomes both full-length articles and Mini Reviews in the wide-ranging field of basic and translational oncology. Furthermore, it frequently presents Special Issues that shed light on current and topical areas in cancer research.
Cancer Letters is highly interested in various fundamental aspects that can cater to a diverse readership. These areas include the molecular genetics and cell biology of cancer, radiation biology, molecular pathology, hormones and cancer, viral oncology, metastasis, and chemoprevention. The journal actively focuses on experimental therapeutics, particularly the advancement of targeted therapies for personalized cancer medicine, such as metronomic chemotherapy.
By publishing groundbreaking research and promoting advancements in cancer treatments, Cancer Letters aims to actively contribute to the fight against cancer and the improvement of patient outcomes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


