深层共晶溶剂中n -酰基腙的设计、硅研究和高效合成:潜在的抗惊厥剂
IF 3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
在药物化学中,寻找有效的癫痫治疗方法仍然至关重要,因为目前的药物往往疗效有限,需要联合使用,并引起不良反应。本研究旨在合成从isatin衍生的n -酰基腙,并探索结合可持续有机合成方法和先进计算工具的方法,以鉴定和开发新的抗癫痫候选药物。采用氯化胆碱和对甲苯磺酸(p-TSA)组成的深共熔溶剂(DES)合成n -酰基腙,开辟了一条高效、选择性强、环境友好的合成途径。在几分钟内合成了18个isatin衍生物,产率从好到好,并且通过适度加热克服了一些底物的低溶解度。DES显示出高可回收性,可重复使用多达四个周期而不损失任何活性。此外,还进行了计算机分析,以评估化合物与GABAA受体之间的配体-受体相互作用,GABAA受体是调节癫痫发作的关键治疗靶点。合成的化合物在GABAA受体结合位点内表现出良好的相互作用,包括氢键、疏水接触和芳香层相互作用。值得注意的是,化合物3d, 3e和30与生物活性相关的关键残基相互作用,与参考配体相比,显示出有希望的结合谱。本研究强化了DES和虚拟筛选作为抗癫痫药物开发的现代有效方法的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
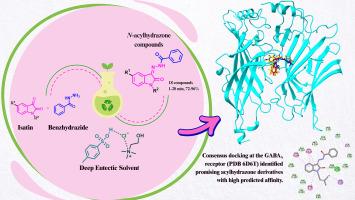
Design, in silico study, and efficient synthesis of N-acylhydrazones in deep eutectic solvents: potential anticonvulsant agents
In medicinal chemistry, the search for effective epilepsy treatments remains critical, as current drugs often show limited efficacy, require combinations, and cause adverse effects. This work aims to synthesize N-acylhydrazones derived from isatin and to explore approaches that combine sustainable organic synthesis methodologies and advanced computational tools for the identification and development of novel antiepileptic drug candidates. The present methods consisted of the synthesis of N-acylhydrazones using a deep eutectic solvent (DES) composed of choline chloride and p-toluene sulfonic acid (p-TSA), which enabled the development of an efficient, selective, and environmentally friendly synthetic route. Eighteen isatin derivatives were synthesized in a few minutes with good to excellent product yields, and the low solubility of some substrates was overcome by moderate heating. DES demonstrated high recyclability, being reused for up to four cycles without any loss of activity. Additionally, in silico analyses were performed to evaluate the ligand-receptor interactions between the compounds and the GABAA receptor, a key therapeutic target for modulating epileptic seizures. The synthesized compounds exhibit favorable interactions within the GABAA receptor binding site, including hydrogen bonds, hydrophobic contacts, and aromatic stacking interactions. Compounds 3d, 3e, and 3o are noteworthy for interacting with key residues associated with biological activity, showing promising binding profiles compared to reference ligands. This study reinforces the potential of DES and virtual screening as modern and effective approaches in the development of antiepileptic drugs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


