线粒体传递在缺血性卒中中的作用:是敌是友?
IF 11.9
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
缺血性中风是全球第二大死亡原因和第三大致残原因。氧化应激引起的能量代谢紊乱和随后的炎症是功能恢复的重要障碍。线粒体的合理分布和功能保存对于维持脑缺血再灌注损伤时的能量稳态和调节炎症反应至关重要。越来越多的证据表明,功能失调的线粒体片段和功能正常的线粒体片段都经历细胞内和细胞间的传递,显著影响脑卒中的预后。本文详细介绍了缺血性卒中中两个截然不同的线粒体过程:功能失调的线粒体片段释放到细胞质或细胞外空间,功能性线粒体进入受损细胞,这两个过程起着双重作用:朋友或敌人。功能失调片段的释放激活下游模式识别受体,包括干扰素基因通路的环状GMP-AMP合成酶刺激因子、黑色素瘤2炎性体中含有3/缺失的NLR家族pyrin结构域和toll样受体,触发神经血管单元内的炎症级联反应,启动导致脑损伤的细胞死亡途径。相反,功能性线粒体的转移通过减轻氧化应激、保持线粒体质量控制、恢复神经元能量代谢、抑制细胞凋亡和维持血脑屏障完整性发挥保护作用。抑制功能失调线粒体片段的释放、增强功能性线粒体转移或应用线粒体移植的治疗方法为改善缺血性卒中的预后提供了巨大的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
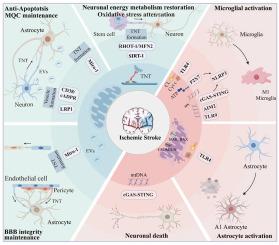
Role of mitochondria transmission in ischemic stroke: Friend or foe?
Ischemic stroke ranks as the second leading cause of mortality and the third disability worldwide. Disruption of energy metabolism and subsequent inflammation driven by oxidative stress constitute significant barriers to functional recovery. Proper distribution and function preservation of mitochondria are essential for maintaining energy homeostasis and modulating the inflammatory response during cerebral ischemia and reperfusion injury. Accumulating evidence indicates that both dysfunctional mitochondrial fragments and functional mitochondria undergo intracellular and intercellular transmission, significantly influencing stroke outcomes. The review details two contrasting mitochondrial processes in ischemic stroke: the release of dysfunctional mitochondrial fragments into the cytoplasm or extracellular space and the entry of functional mitochondria into damaged cells, which plays a dual role: friend or foe. The release of dysfunctional fragments activates downstream pattern recognition receptors, including the cyclic GMP-AMP synthase–stimulator of interferon genes pathway, NLR family pyrin domain containing 3/absent in melanoma 2 inflammasome, and Toll-like receptors, triggering inflammatory cascades within the neurovascular unit and initiating cell death pathways contributing to cerebral injury. In contrast, the transfer of functional mitochondria plays a protective role by attenuating oxidative stress, preserving mitochondrial quality control, restoring neuronal energy metabolism, inhibiting apoptosis, and maintaining blood-brain barrier integrity. Therapeutic approaches that inhibit the release of dysfunctional mitochondrial fragments, enhance functional mitochondria transfer, or apply mitochondrial transplantation offer significant potential for improving outcomes in ischemic stroke.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Redox Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-
CiteScore
19.90
自引率
3.50%
发文量
318
审稿时长
25 days
期刊介绍:
Redox Biology is the official journal of the Society for Redox Biology and Medicine and the Society for Free Radical Research-Europe. It is also affiliated with the International Society for Free Radical Research (SFRRI). This journal serves as a platform for publishing pioneering research, innovative methods, and comprehensive review articles in the field of redox biology, encompassing both health and disease.
Redox Biology welcomes various forms of contributions, including research articles (short or full communications), methods, mini-reviews, and commentaries. Through its diverse range of published content, Redox Biology aims to foster advancements and insights in the understanding of redox biology and its implications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


