具有双重抑制EGFR和COX-2治疗癌症和炎症性疾病的新型1,5-二芳基吡唑类羧酰胺的设计、合成和生物学评价
IF 2.2
4区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
本研究提出了一系列双靶点表皮生长因子受体(EGFR)和环氧化酶-2 (COX-2)抑制剂作为抗癌和抗炎药物。这些新型抑制剂是根据已知的具有不同化学结构的选择性EGFR/COX-2抑制剂的关键药效特征设计的,并根据合理的标准选择各种取代基。许多新化合物表现出良好的抗增殖活性和EGFR/COX-2抑制活性。其中10a对肿瘤细胞系的抑制作用最强,对白血病、宫颈癌和胰腺癌的IC50值分别为6.7、9.0和13.0 μM。在人PC12正常细胞株的细胞活力实验中,优选化合物10a显示出较低的细胞毒性(IC50 = 77.4 μM, SI = 8.6, 11.5, 5.9),可能是一种有效的、选择性的、安全的抗肿瘤药物。对EGFR (IC50 = 6.0 μM)和COX-2 (IC50 = 50 μM, SI = 3.8)具有较高的抑制活性。机制研究表明,10a增加了S期的细胞数量,并主要在此期诱导细胞周期阻滞。此外,10a处理后,凋亡的HeLa细胞增加。此外,8d被确定为最有效和选择性的COX-2抑制剂,IC50值为12.5 μM, SI为16。分子对接研究表明,EGFR和COX-2结合位点的10a和8d采用的取向与配体抑制剂的取向和契合方式相似,支持实验结果。本文章由计算机程序翻译,如有差异,请以英文原文为准。
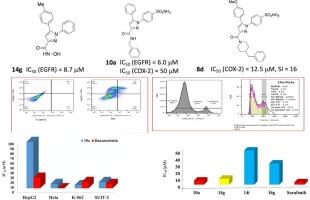
Design, synthesis, and biological evaluation of novel 1,5-diarylpyrazole carboxamides with dual inhibition of EGFR and COX-2 for the treatment of cancer and inflammatory diseases
This study presents a series of dual-target epidermal growth factor receptor (EGFR) and cyclooxygenase-2 (COX-2) inhibitors developed as anticancer and anti-inflammatory agents. These novel inhibitors were designed based on key pharmacophoric features of known selective EGFR/COX-2 inhibitors with distinct chemical structures, and various substituents were selected according to rational criteria. Many of the novel compounds exhibited excellent antiproliferative activities and EGFR/COX-2 inhibitory activity. Among them, 10a exhibited the highest potency against cancer cell lines, with IC50 values of 6.7, 9.0, and 13.0 μM against leukemia, cervical cancer, and pancreatic cancer cell lines, respectively. Moreover, the optimal compound 10a was tested in a cell viability assay using human PC12 normal cell line and exhibited low cytotoxicity (IC50 = 77.4 μM, SI = 8.6, 11.5, 5.9) and it might be used as a potent, selective and safe antitumor agent. It also exhibited high inhibitory activity against EGFR (IC50 = 6.0 μM) and COX-2 (IC50 = 50 μM, SI = 3.8). Mechanistic studies revealed that 10a increased the cell population in the S phase and induced cell cycle arrest mainly in this phase. Moreover, an increase in apoptotic HeLa cells was observed following treatment with 10a. In addition, 8d was identified as the most potent and selective COX-2 inhibitor, with an IC50 value of 12.5 μM and SI of 16. Molecular docking studies demonstrated that 10a and 8d adopted orientations in the EGFR and COX-2 binding sites are oriented and fit in a similar manner to those of ligand inhibitors, supporting the experimental results.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
5.70
自引率
3.70%
发文量
463
审稿时长
27 days
期刊介绍:
Bioorganic & Medicinal Chemistry Letters presents preliminary experimental or theoretical research results of outstanding significance and timeliness on all aspects of science at the interface of chemistry and biology and on major advances in drug design and development. The journal publishes articles in the form of communications reporting experimental or theoretical results of special interest, and strives to provide maximum dissemination to a large, international audience.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


