机器学习驱动的NSC828779作为炎性疾病多机制NLRP3炎性体抑制剂的发现
IF 6.3
2区 医学
Q1 BIOLOGY
引用次数: 0
摘要
NLRP3炎症小体是先天免疫反应的关键调节因子,也是炎症驱动疾病的一个有希望的治疗靶点。本研究旨在利用人工智能引导的硅技术与NCI-60高通量分析相结合,鉴定有效的自然激发小分子。我们开发了一个机器学习驱动的平台,结合药效团建模、分子对接、MDS和rnn来优先考虑候选化合物。其中,NSC828779作为先导支架出现,与NLRP3的atp结合位点具有高结合亲和力,与已知抑制剂相比具有更好的相互作用能和稳定性。NLRP3 (- 10.5 kcal/mol)、caspase-1 (- 8.6 kcal/mol)和ASC (- 8.5 kcal/mol)的对接评分最高,优于MCC950、格列本脲和其他参比化合物。MDS证实了NLRP3-ASC-caspase-1复合物的稳定性,RMSD和RMSF分析也显示了增强的构象完整性。ADMET分析预测了良好的药物相似性、溶解度、中等亲脂性和低毒性。从机制上看,NSC828779可能通过破坏蛋白相互作用、抑制NF-κB信号传导、诱导自噬而作为多机制的NLRP3抑制剂。这些结果确立了NSC828779作为治疗炎症相关疾病的有希望的候选药物,并强调了人工智能驱动的药物发现平台在识别新型炎症小体靶向治疗方面的实用性。进一步的体外和体内验证是必要的,以支持其临床发展。本文章由计算机程序翻译,如有差异,请以英文原文为准。
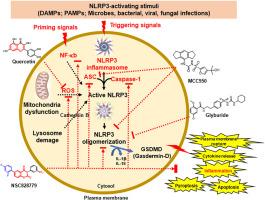
Machine learning–driven discovery of NSC828779 as a multi-mechanistic NLRP3 inflammasome inhibitor for inflammatory diseases
The NLRP3 inflammasome is a key regulator of the innate immune response and a promising therapeutic target in inflammation-driven diseases. This study aimed to identify potent nature inspired small molecules using AI-guided in silico techniques integrated with NCI-60 high-throughput assays. We developed a machine learning–driven platform that combines pharmacophore modeling, molecular docking, MDS, and RNNs to prioritize candidate compounds. Among these, NSC828779 emerged as a lead scaffold, demonstrating high binding affinity to the ATP-binding site of NLRP3 and superior interaction energy and stability compared to known inhibitors. Docking scores were strongest for NLRP3 (−10.5 kcal/mol), caspase-1 (−8.6 kcal/mol), and ASC (−8.5 kcal/mol), outperforming MCC950, glyburide, and other reference compounds. MDS confirmed the stability of the NLRP3–ASC–caspase-1 complex, supported by RMSD and RMSF analyses showing enhanced conformational integrity. ADMET profiling predicted favorable drug-likeness, solubility, moderate lipophilicity, and low toxicity. Mechanistically, NSC828779 may act as a multi-mechanistic NLRP3 inhibitor by disrupting protein–protein interactions, inhibiting NF-κB signaling, and inducing autophagy. These results establish NSC828779 as a promising candidate for treating inflammation-related disorders and underscore the utility of AI-driven drug discovery platforms in identifying novel inflammasome-targeted therapeutics. Further in vitro and in vivo validation is warranted to support its clinical development.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Computers in biology and medicine
工程技术-工程:生物医学
CiteScore
11.70
自引率
10.40%
发文量
1086
审稿时长
74 days
期刊介绍:
Computers in Biology and Medicine is an international forum for sharing groundbreaking advancements in the use of computers in bioscience and medicine. This journal serves as a medium for communicating essential research, instruction, ideas, and information regarding the rapidly evolving field of computer applications in these domains. By encouraging the exchange of knowledge, we aim to facilitate progress and innovation in the utilization of computers in biology and medicine.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


