通过HNF4A/PPARA/LDLR高通量筛选揭示芍药苷对apo2缺乏性高甘油三酯血症的疗效
IF 5.6
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
高甘油三酯血症(HTG)是心血管疾病、脂肪肝和急性胰腺炎的重要危险因素,但由于目前治疗方案的局限性,仍然是一个治疗挑战。为了解决这一未满足的临床需求,我们筛选了一个初始浓度为100 μM的天然小分子文库,使用CRISPR/ cas9生成的载脂蛋白C2 (apoc2)敲除斑马鱼模型来确定有效的HTG治疗候选药物,该模型类似于人类脂质代谢紊乱。基于表型的筛选从351种化合物中鉴定出芍药苷(PAE)是一种有效的甘油三酯降低剂。脂质组学分析显示,PAE通过β-氧化和脂质吞噬促进甘油三酯脂解。机制研究表明,PAE在apoc2突变体中上调过氧化物酶体增殖激活受体α (ppara)和脂蛋白受体(ldlr)。在油酸诱导的Huh7人肝细胞中,PAE减少细胞内脂滴积累,显著上调PPARA和LDLR的表达,表明肝细胞对富含甘油三酯的脂蛋白的摄取和氧化分解代谢增强。进一步研究发现,PAE上调PPARA上游关键转录因子HNF4A的表达。HNF4A抑制剂BI-6015完全消除了PAE的甘油三酯降低作用,提示通过HNF4A- ppara - ldlr轴介导。这些发现表明,PAE通过靶向HNF4A-PPARA-LDLR通路的新机制,有望成为HTG的治疗候选药物。我们的工作不仅确定了HTG治疗的潜在先导化合物,而且支持斑马鱼模型作为发现靶向肝脂质代谢途径药物的有效平台。本文章由计算机程序翻译,如有差异,请以英文原文为准。
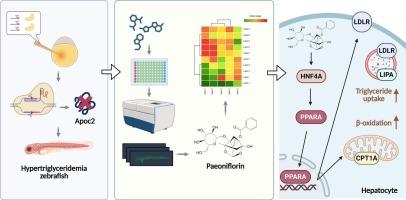
High-throughput screening reveals paeoniflorin’s efficacy against Apoc2-deficient hypertriglyceridemia via HNF4A/PPARA/LDLR
Hypertriglyceridemia (HTG) is a significant risk factor for cardiovascular disease, fatty liver, and acute pancreatitis, yet remains a therapeutic challenge due to limitations of current treatment options. To address this unmet clinical need, we screened a natural small-molecule library at an initial concentration of 100 μM to identify effective HTG therapeutic candidates using a CRISPR/Cas9-generated apolipoprotein C2 (apoc2) knockout zebrafish model that resembles human lipid metabolism disorders. Phenotype-based screening identified paeoniflorin (PAE) from 351 compounds as a potent triglyceride-lowering agent. Lipidomics analysis revealed PAE promoted triglyceride lipolysis by β-oxidation and lipophagy. Mechanistic studies demonstrated PAE upregulates peroxisome proliferator-activated receptor α (ppara) and lipoprotein receptor (ldlr) in apoc2 mutants. In oleic acid-induced Huh7 human hepatocytes, PAE reduces intracellular lipid droplet accumulation and significantly upregulated PPARA and LDLR expression, indicating enhanced hepatocellular uptake and oxidative catabolism of triglyceride-rich lipoproteins. Further investigation revealed that PAE upregulates the expression of hepatocyte nuclear factor 4 α (HNF4A), a key upstream transcription factor of PPARA. The HNF4A inhibitor BI-6015 completely abolished PAE’s triglyceride-lowering effects, suggesting mediation through the HNF4A-PPARA-LDLR axis. These findings establish PAE as a promising therapeutic candidate for HTG through a novel mechanism targeting the HNF4A-PPARA-LDLR pathway. Our work not only identifies a potential lead compound for HTG treatment but also supports the zebrafish model as an effective platform for discovering drugs targeting hepatic lipid metabolic pathways.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


