HIF-1α/A2BAR信号通路通过阻止巨噬细胞向肌成纤维细胞转变,减轻缺血再灌注损伤后肾纤维化
IF 5.6
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
缺氧或缺血条件下缺氧诱导因子-1α (HIF-1α)的激活在急性肾损伤(AKI)到慢性肾脏疾病(CKD)的进展中起着至关重要的作用。炎症性巨噬细胞可能参与肾脏纤维化,巨噬细胞向肌成纤维细胞转化(MMT)是肾脏纤维化的重要因素。本研究探讨HIF-1α在缺血再灌注损伤(IRI)后肾纤维化发展过程中调控MMT的作用。利用野生型(WT)和HIF-1α-敲低小鼠的aki向ckd过渡模型,我们评估了肾脏纤维化、巨噬细胞浸润和MMT。通过调节腺苷A2B受体(A2BAR)活性来评估HIF-1α/A2BAR信号通路对MMT的影响。在WT小鼠中,iri诱导的肾纤维化与巨噬细胞浸润和MMT标记物共表达(F4/80+ -α-SMA +)增加以及HIF-1α和A2BAR通路的激活有关。HIF-1α下调后,巨噬细胞浸润和MMT明显增加,肾纤维化明显加重。hif -1α-敲低小鼠用拮抗剂MRS1754抑制A2BAR信号传导进一步增加巨噬细胞浸润和MMT,加重iri后肾纤维化。相比之下,使用激动剂BAY60-6583激活A2BAR信号可显著降低巨噬细胞浸润和MMT,有效减轻肾纤维化。这项研究强调了MMT在IRI后肾纤维化中的关键作用,并表明HIF-1α/A2BAR信号通路通过调节巨噬细胞浸润和MMT来减轻纤维化,为aki到ckd进展的机制提供了新的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
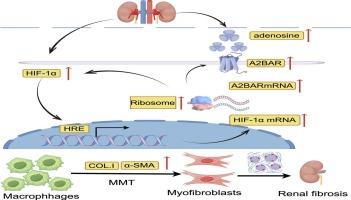
HIF-1α/A2BAR signalling pathway alleviates kidney fibrosis after ischemia–reperfusion injury by preventing macrophage-to-myofibroblast transition
The activation of hypoxia-inducible factor-1α (HIF-1α) under hypoxic or ischemic conditions plays a crucial in the progression from acute kidney injury (AKI) to chronic kidney disease (CKD). Inflammatory macrophages are potentially involved in kidney fibrosis, and the macrophage-to-myofibroblast transition (MMT) is a significant contributor to renal fibrosis. This study investigates the role of HIF-1α in modulating MMT during renal fibrosis development post-ischemia–reperfusion injury (IRI). Using a model of AKI-to-CKD transition in wild-type (WT) and HIF-1α- knockdown mice, we evaluated kidney fibrosis, macrophage infiltration, and MMT. The influence of the HIF-1α/A2BAR signalling pathway on MMT was assessed by modulating adenosine A2B receptor (A2BAR) activity. In WT mice, IRI-induced renal fibrosis was associated with increased macrophage infiltration and MMT marker co-expression (F4/80+–α-SMA+), alongside activation of the HIF-1α and A2BAR pathways. Following HIF-1α knockdown, macrophage infiltration and MMT increased significantly, accompanied by a marked aggravation of renal fibrosis. Inhibition of A2BAR signalling with the antagonist MRS1754 in HIF-1α-knockdown mice further increased macrophage infiltration and MMT, aggravating renal fibrosis post-IRI. In contrast, activation of A2BAR signalling with the agonist BAY60-6583 markedly decreased macrophage infiltration and MMT, effectively mitigating renal fibrosis. This study underscores the critical role of MMT in renal fibrosis after IRI and suggests that the HIF-1α/A2BAR signalling pathway mitigates fibrosis by modulating macrophage infiltration and MMT, providing new insights into the mechanisms underlying the AKI-to-CKD progression.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


