Asterosaponin CN-3通过调控miR-4465/HMGA1/NF-κB信号通路,抑制胶质瘤细胞的迁移和侵袭,发挥抗胶质瘤作用。
IF 5.6
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
恶性胶质瘤是一种毁灭性的肿瘤,通常在诊断后1 年内导致患者死亡。弥漫性侵袭是肿瘤治愈的主要障碍,它使肿瘤无法完成手术切除和化疗、放疗。因此,迫切需要开发抑制胶质瘤浸润性生长的新型化疗药物。新几内亚Culcita novaeguineae-3 (CN-3)是一种海洋来源的甾体皂苷,对胶质瘤细胞具有显著的细胞毒性。本研究旨在证实CN-3对胶质瘤的作用,并进一步阐明其作用机制。实验结果表明,CN-3在体外和体内均能显著抑制胶质瘤细胞的增殖、侵袭和迁移。CN-3可下调高迁移率组蛋白A1 (HMGA1)的表达,抑制胶质瘤细胞的浸润性生长。此外,HMGA1敲除胶质瘤细胞的转移性显著降低。此外,miR-4465被证实靶向并降低HMGA1的表达。此外,CN-3可以上调细胞中miR-4465的表达,从而抑制胶质瘤细胞的迁移和侵袭。这些结果阐明了CN-3这种来自海洋的甾体皂苷可能通过miRNA-4465/HMGA1/NF-κB通路抑制胶质瘤细胞侵袭行为的机制,也为CN-3作为化疗药物预防胶质瘤侵袭性生长提供了前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
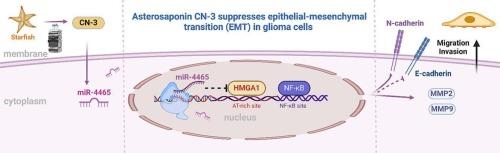
Asterosaponin CN-3 performs an anti-glioma effect by inhibiting the migration and invasion of glioma cells through the regulation of miR-4465/HMGA1/NF-κB signaling pathway
Malignant gliomas are devastating tumors that frequently kill patients within 1 year of diagnosis. Diffuse invasion is a major obstacle to cure, which allows tumor to escape from complete surgical resection as well as chemotherapy and radiotherapy. Therefore, the development of new chemotherapeutic agent that inhibiting the infiltrative growth of glioma is urgently needed. Culcita novaeguineae-3 (CN-3) is a marine-derived steroidal saponin which exhibit significant cytotoxicity to glioma cells. This study was designed to confirm the effect of CN-3 on glioma and to further clarify its mechanism of action. The experimental results showed that CN-3 could significantly inhibit the proliferation, invasion and migration of glioma cells in vitro and in vivo. CN-3 could down-regulate the expression of high mobility group protein A1 (HMGA1), to inhibit the infiltrative growth of glioma cells. Furthermore, the metastatic mobility was significantly reduced in HMGA1 knockout glioma cells. Moreover, miR-4465 was confirmed to target and reduce HMGA1 expression. In addition, CN-3 could upregulate the expression of miR-4465 in cells, thereby inhibiting migration and invasion of glioma cells. These results elucidate the mechanism by which CN-3, a steroidal saponin from the sea, may inhibit the invasive behavior of glioma cells through the miRNA-4465/HMGA1/NF-κB pathway, which also raise the prospect of using CN-3 as a chemotherapeutic agent to prevent glioma invasive growth.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochemical pharmacology
医学-药学
CiteScore
10.30
自引率
1.70%
发文量
420
审稿时长
17 days
期刊介绍:
Biochemical Pharmacology publishes original research findings, Commentaries and review articles related to the elucidation of cellular and tissue function(s) at the biochemical and molecular levels, the modification of cellular phenotype(s) by genetic, transcriptional/translational or drug/compound-induced modifications, as well as the pharmacodynamics and pharmacokinetics of xenobiotics and drugs, the latter including both small molecules and biologics.
The journal''s target audience includes scientists engaged in the identification and study of the mechanisms of action of xenobiotics, biologics and drugs and in the drug discovery and development process.
All areas of cellular biology and cellular, tissue/organ and whole animal pharmacology fall within the scope of the journal. Drug classes covered include anti-infectives, anti-inflammatory agents, chemotherapeutics, cardiovascular, endocrinological, immunological, metabolic, neurological and psychiatric drugs, as well as research on drug metabolism and kinetics. While medicinal chemistry is a topic of complimentary interest, manuscripts in this area must contain sufficient biological data to characterize pharmacologically the compounds reported. Submissions describing work focused predominately on chemical synthesis and molecular modeling will not be considered for review.
While particular emphasis is placed on reporting the results of molecular and biochemical studies, research involving the use of tissue and animal models of human pathophysiology and toxicology is of interest to the extent that it helps define drug mechanisms of action, safety and efficacy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


